Lab Anim Res.
2011 Jun;27(2):117-126. 10.5625/lar.2011.27.2.117.
Effects of Steaming Time and Frequency for Manufactured Red Liriope platyphylla on the Insulin Secretion Ability and Insulin Receptor Signaling Pathway
- Affiliations
-
- 1College of Natural Resources and Life Science, Pusan National University, Miryang, Republic of Korea. dyhwang@pusan.ac.kr
- 2College of Human Ecology, Pusan National University, Pusan, Republic of Korea.
- 3Noghyup Sandong Processing Plant, Miryang, Republic of Korea.
- 4Department of Animal Science & Technology, Gyeongnam National University of Science and Technology, Republic of Korea.
- KMID: 2053660
- DOI: http://doi.org/10.5625/lar.2011.27.2.117
Abstract
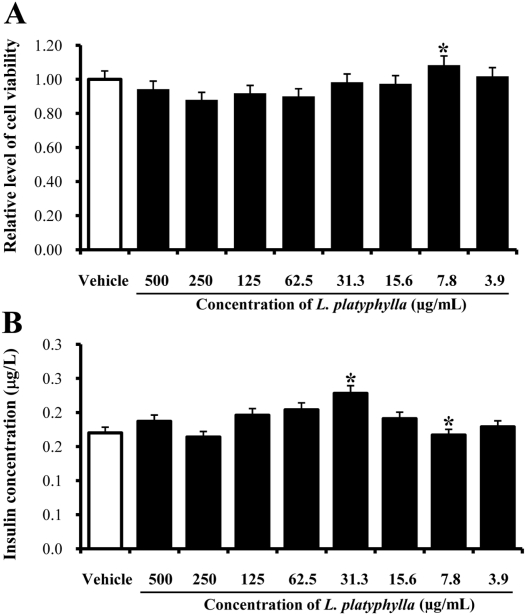
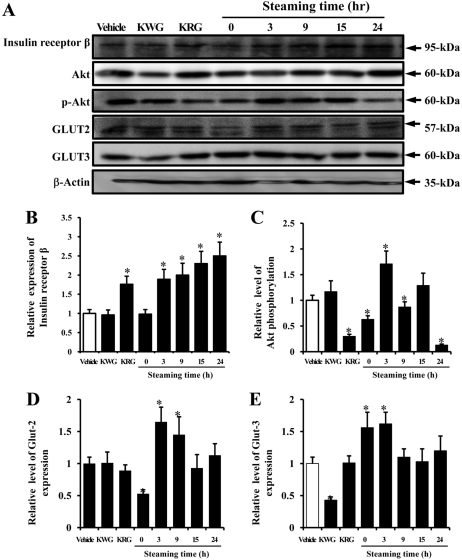
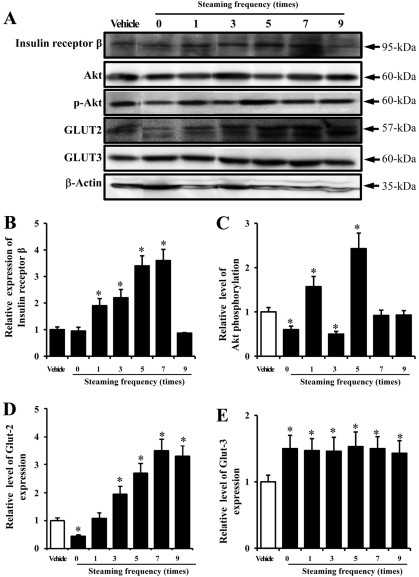
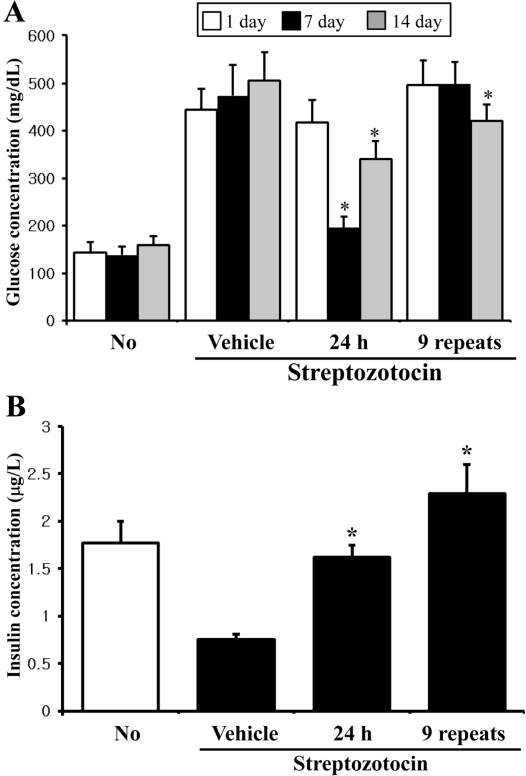
- In oriental medicine, Liriope platyphylla (LP) has long been regarded as a curative herb useful for the treatment of diabetes, asthma, and neurodegenerative disorders. The principal objective of this study was to assess the effects of steaming time and frequency for manufactured Red LP (RLP) on insulin secretion ability and insulin receptor signaling pathway. To achieve our goal, several types of LPs manufactured under different conditions were applied to INS cells and streptozotocin (STZ)-induced diabetic ICR mice, after which alterations in insulin concentrations were detected in the culture supernatants and sera. The optimal concentration for the investigation of insulin secretion ability was found to be 50 ug/mL of LP. At this concentration, maximum insulin secretion was observed in the INS cells treated with LP extract steamed for 3 h (3-SLP) with two repeated steps (3 h steaming and 24 h air-dried) carried out 9 times (9-SALP); no significant changes in viability were detected in any of the treated cells. Additionally, the expression and phosphorylation levels of most components in the insulin receptor signaling pathway were increased significantly in the majority of cells treated with steaming-processed LP as compared to the cells treated with LP prepared without steaming. With regard to glucose transporter (GLUT) expression, alterations of steaming time induced similar responses on the expression levels of GLUT-2 and GLUT-3. However, differences in steaming frequency were also shown to induce dose-dependent responses in the expression level of GLUT-2 only; no significant differences in GLUT-3 expression were detected under these conditions. Furthermore, these responses observed in vitro were similarly detected in STZ-induced diabetic mice. 24-SLP and 9-SALP treatment applied for 14 days induced the down-regulation of glucose concentration and upregulation of insulin concentration. Therefore, these results indicated that the steaming processed LP may contribute to the relief of diabetes symptoms and should be regarded as an excellent candidate for a diabetes treatment.
Keyword
MeSH Terms
-
Animals
Asthma
Down-Regulation
Glucose
Glucose Transport Proteins, Facilitative
Insulin
Medicine, East Asian Traditional
Mice
Mice, Inbred ICR
Neurodegenerative Diseases
Phosphorylation
Receptor, Insulin
Steam
Streptozocin
Up-Regulation
Glucose
Glucose Transport Proteins, Facilitative
Insulin
Receptor, Insulin
Steam
Streptozocin
Figure
Reference
-
1. Lee YC, Lee JC, Seo YB, Kook YB. Liriopis tuber inhibit OVA-induced airway inflammation and bronchial hyperresponsiveness in murine model of asthma. J Ethnopharmacol. 2005; 101(1-3):144–152. PMID: 15982838.
Article2. Huh MK, Huh HW, Choi JS, BK Lee. Genetic diversity and population structure of Liriope platyphylla (Liliaceae) in Korea. J Life Sci. 2007; 17(3):328–333.3. Choi SB, Wha JD, Park S. The insulin sensitizing effect of homoisoflavone-enriched fraction in Liriope platyphylla Wang et Tang via PI3-kinase pathway. Life Sci. 2004; 75(22):2653–2664. PMID: 15369701.4. Jeong S, Chae K, Jung YS, Rho YH, Lee J, Ha J, Yoon KH, Kim GC, Oh KS, Shin SS, Yoon M. The Korean traditional medicine Gyeongshingangjeehwan inhibits obesity through the regulation of leptin and PPARalpha action in OLETF rats. J Ethnopharmacol. 2008; 119(2):245–251. PMID: 18674606.5. Kim SW, Chang IM, Oh KB. Inhibition of the bacterial surface protein anchoring transpeptidase sortase by medicinal plants. Biosci Biotechnol Biochem. 2002; 66(12):2751–2754. PMID: 12596883.
Article6. Hur J, Lee P, Kim J, Kim AJ, Kim H, Kim SY. Induction of nerve growth factor by butanol fraction of Liriope platyphylla in C6 and primary astrocyte cells. Biol Pharm Bull. 2004; 27(8):1257–1260. PMID: 15305032.7. Hur J, Lee P, Moon E, Kang I, Kim SH, Oh MS, Kim SY. Neurite outgrowth induced by spicatoside A, a steroidal saponin, via the tyrosine kinase A receptor pathway. Eur J Pharmacol. 2009; 620(1-3):9–15. PMID: 19695245.
Article8. Lee YK, Kim JE, Nam SH, Goo JS, Choi SI, Choi YH, Bae CJ, Woo JM, Cho JS, Hwang DY. Differential regulation of the biosynthesis of glucose transporters by the PI3-K and MAPK pathways of insulin signaling by treatment with novel compounds from Liriope platyphylla. Int J Mol Med. 2011; 27(3):319–327. PMID: 21165549.
Article9. Kim K, Kim HY. Korean red ginseng stimulates insulin release from isolated rat pancreatic islets. J Ethnopharmacol. 2008; 120(2):190–195. PMID: 18773949.
Article10. Lu JM, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009; 7(3):293–302. PMID: 19601854.
Article11. Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng). J Pharm Pharmacol. 2006; 58(8):1007–1019. PMID: 16872547.12. Kiefer D, Pantuso T. Panax ginseng. Am Fam Physician. 2003; 68(8):1539–1542. PMID: 14596440.13. Baek NI, Kim DS, Lee YH, Park JD, Lee CB, Kim SI. Ginsenoside Rh4, a genuine dammarane glycoside from Korean red ginseng. Planta Med. 1996; 62(1):86–87. PMID: 8720394.
Article14. Yun TK, Lee YS, Kwon KH, Choi KJ. Saponin contents and anticarcinogenic effects of ginseng depending on types and ages in mice. Zhongguo Yao Li Xue Bao. 1996; 17(4):293–298. PMID: 9812705.15. Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997; 275(5300):661–665. PMID: 9005851.
Article16. Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem. 2002; 277(42):39684–39695. PMID: 12138094.
Article17. Mun JH, Lee SG, Kim DH, Jung JW, Lee SJ, Yoon BH, Shin BY, Kim SH, Ryu JH. Neurotrophic factors mediate enhancing property of ethanolic extract of Liriope platyphylla in mice. J Appl Pharmacol. 2007; 15:83–88.18. Kimura M, Waki I, Chujo T, Kikuchi T, Hiyama C, Yamazaki K, Tanaka O. Effects of hypoglycemic components in ginseng radix on blood insulin level in alloxan diabetic mice and on insulin release from perfused rat pancreas. J Pharmacobiodyn. 1981; 4(6):410–417. PMID: 7026762.
Article19. Waki I, Kyo H, Yasuda M, Kimura M. Effects of a hypoglycemic component of ginseng radix on insulin biosynthesis in normal and diabetic animals. J Pharmacobiodyn. 1982; 5(8):547–554. PMID: 6759629.
Article20. Chung SH, Choi CG, Park SH. Comparisons between white ginseng radix and rootlet for antidiabetic activity and mechanism in KKAy mice. Arch Pharm Res. 2010; 24(3):214–218. PMID: 11440080.
Article21. Dey L, Xie JT, Wang A, Wu J, Maleckar SA, Yuan CS. Anti-hyperglycemic effects of ginseng: comparison between root and berry. Phytomedicine. 2003; 10(6-7):600–605. PMID: 13678250.
Article22. Kim JH, Hahm DH, Yang DC, Kim JH, Lee MHJ, Shim I. Effect of crude saponin of Korean red ginseng on high-fat diet-induced obesity in the rat. J Pharmacol Sci. 2005; 97(1):124–131. PMID: 15655288.
Article23. Liu TP, Liu IM, Cheng JT. Improvement of insulin resistance by Panax ginseng in fructose-rich chow-fed rats. Horm Metab Res. 2005; 37(3):146–151. PMID: 15824968.24. Vuksan V, Sung MK, Sievenpiper JL, Stavro PM, Jenkins AL, Di Buono M, Lee KS, Leiter LA, Nam KY, Arnason JT, Choi M, Naeem A. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis. 2008; 18(1):46–56. PMID: 16860976.25. Su CF, Cheng JT, Liu IM. Increase of acetylcholine release by Panax ginseng root enhances insulin secretion in Wistar rats. Neurosci Lett. 2007; 412(2):101–104. PMID: 17123721.
Article26. Fritsche L, Weigert C, Häring HU, Lehmann R. How insulin receptor substrate proteins regulate the metabolic capacity of the liver-implications for health and disease. Curr Med Chem. 2008; 15(13):1316–1329. PMID: 18537611.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Precautionary effects of Red Liriope platyphylla on NGF secretion and Abeta42 deposition under the preclinical stage of Alzheimer's disease in Tg2576 mice
- Effects of Red Liriope platyphylla on NGF secretion ability, NGF receptor signaling pathway and gamma-secretase components in NSE/hAPPsw transgenic mice expressing Alzheimer's Disease
- Red Liriope platyphylla stimulated the insulin secretion through the regulation of calcium concentration in rat insulinoma cells and animal models
- Toxicity of red Liriope platyphylla manufactured by steaming process on liver and kidney organs of ICR mice
- Rho-kinase and Insulin Signaling