Tuberc Respir Dis.
2019 Jan;82(1):42-52. 10.4046/trd.2017.0111.
The Effects of Retinoic Acid and MAPK Inhibitors on Phosphorylation of Smad2/3 Induced by Transforming Growth Factor β1
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Institute of Chest Diseases, Severance Hospital, Younsei University Health System, Yonsei University College of Medicine, Seoul, Korea. pms70@yuhs.ac
- 2Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- KMID: 2441729
- DOI: http://doi.org/10.4046/trd.2017.0111
Abstract
- BACKGROUND
Transforming growth factor β (TGF-β), retinoic acid (RA), p38 mitogen-activated protein kinase (MAPK), and MEK signaling play critical roles in cell differentiation, proliferation, and apoptosis. We investigated the effect of RA and the role of these signaling molecules on the phosphorylation of Smad2/3 (p-Smad2/3) induced by TGF-β1.
METHODS
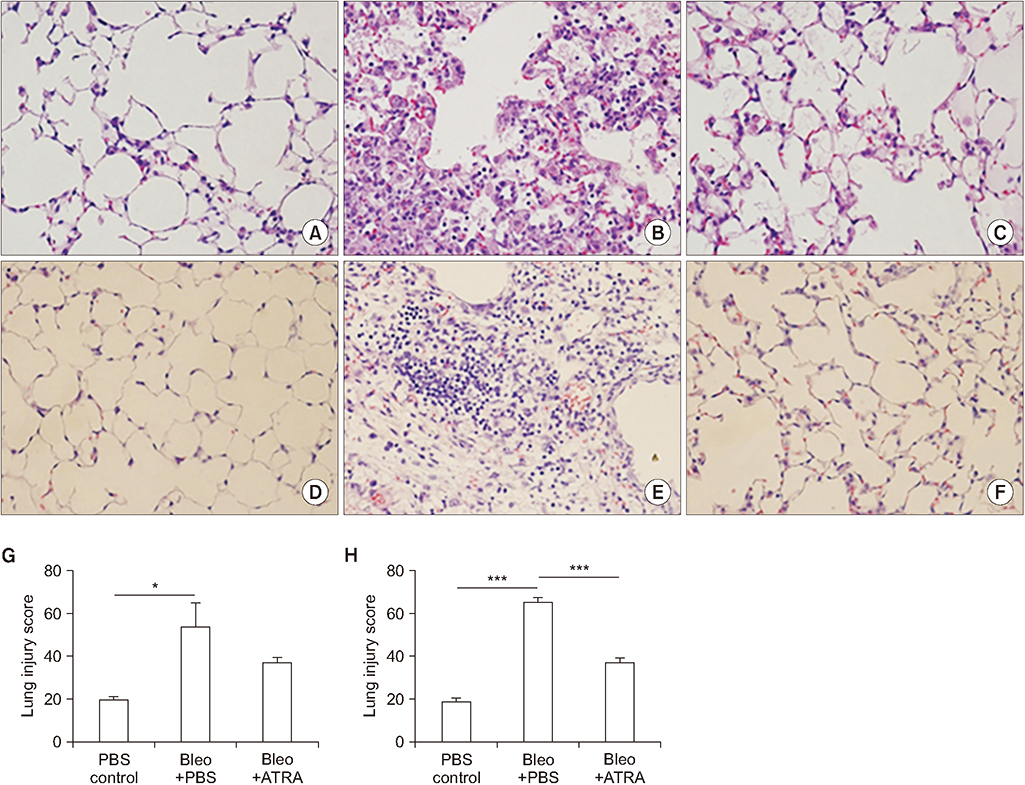
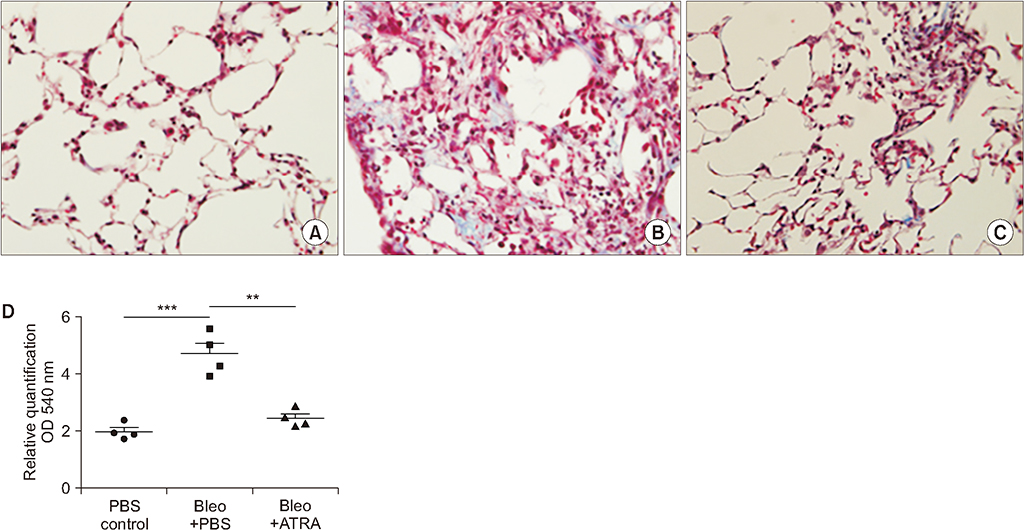
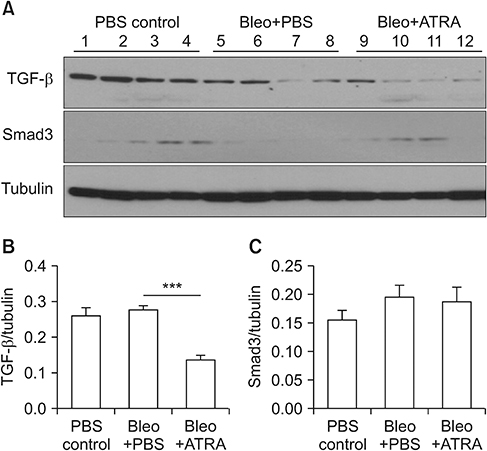
A549 epithelial cells and CCD-11Lu fibroblasts were incubated and stimulated with or without all-trans RA (ATRA) and TGF-β1 and with MAPK or MEK inhibitors. The levels of p-Smad2/3 were analyzed by western blotting. For animal models, we studied three experimental mouse groups: control, bleomycin, and bleomycin+ATRA group. Changes in histopathology, lung injury score, and levels of TGF-β1 and Smad3 were evaluated at 1 and 3 weeks.
RESULTS
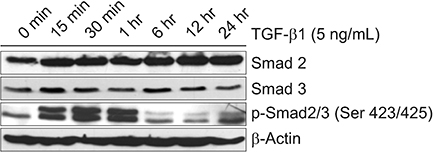
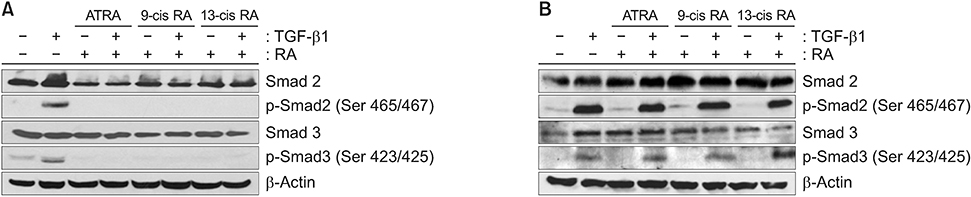
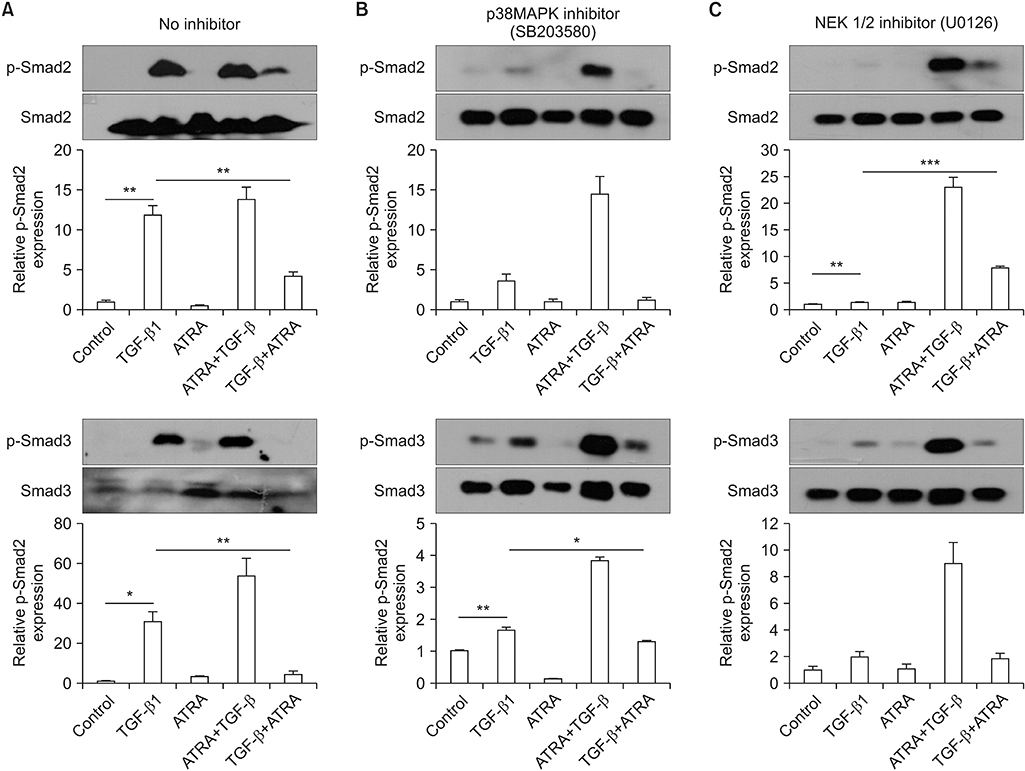
When A549 cells were pre-stimulated with TGF-β1 prior to RA treatment, RA completely inhibited the p-Smad2/3. However, when A549 cells were pre-treated with RA prior to TGF-β1 stimulation, RA did not completely suppress the p-Smad2/3. When A549 cells were pre-treated with MAPK inhibitor, TGF-β1 failed to phosphorylate Smad2/3. In fibroblasts, p38 MAPK inhibitor suppressed TGF-β1-induced p-Smad2. In a bleomycin-induced lung injury mouse model, RA decreased the expression of TGF-β1 and Smad3 at 1 and 3 weeks.
CONCLUSION
RA had inhibitory effects on the phosphorylation of Smad induced by TGF-β1 in vitro, and RA also decreased the expression of TGF-β1 at 1 and 3 weeks in vivo. Furthermore, pre-treatment with a MAPK inhibitor showed a preventative effect on TGF-β1/Smad phosphorylation in epithelial cells. As a result, a combination of RA and MAPK inhibitors may suppress the TGF-β1-induced lung injury and fibrosis.
Keyword
MeSH Terms
-
Animals
Apoptosis
Bleomycin
Blotting, Western
Cell Differentiation
Epithelial Cells
Fibroblasts
Fibrosis
In Vitro Techniques
Lung Injury
Mice
Mitogen-Activated Protein Kinase Kinases
Mitogen-Activated Protein Kinases
Models, Animal
p38 Mitogen-Activated Protein Kinases
Phosphorylation*
Protein Kinases
Smad Proteins
Transforming Growth Factor beta
Transforming Growth Factors*
Tretinoin*
Bleomycin
Mitogen-Activated Protein Kinase Kinases
Mitogen-Activated Protein Kinases
Protein Kinases
Smad Proteins
Transforming Growth Factor beta
Transforming Growth Factors
Tretinoin
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
1. Selman M, King TE, Pardo A. American Thoracic Society. European Respiratory Society. American College of Chest Physicians. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001; 134:136–151.
Article2. Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001; 345:517–525.
Article3. Lepparanta O, Sens C, Salmenkivi K, Kinnula VL, Keski-Oja J, Myllarniemi M, et al. Regulation of TGF-β storage and activation in the human idiopathic pulmonary fibrosis lung. Cell Tissue Res. 2012; 348:491–503.
Article4. Gu L, Zhu YJ, Guo ZJ, Xu XX, Xu WB. Effect of IFN-gamma and dexamethasone on TGF-beta1-induced human fetal lung fibroblast-myofibroblast differentiation. Acta Pharmacol Sin. 2004; 25:1479–1488.5. Wen FQ, Liu X, Kobayashi T, Abe S, Fang Q, Kohyama T, et al. Interferon-gamma inhibits transforming growth factor-beta production in human airway epithelial cells by targeting Smads. Am J Respir Cell Mol Biol. 2004; 30:816–822.6. Wang J, Yang Y, Xu J, Lin X, Wu K, Yu M. Pirfenidone inhibits migration, differentiation, and proliferation of human retinal pigment epithelial cells in vitro. Mol Vis. 2013; 19:2626–2635.7. Zhang YE. Non-Smad pathways in TGF-β signaling. Cell Res. 2009; 19:128–139.
Article8. Pendaries V, Verrecchia F, Michel S, Mauviel A. Retinoic acid receptors interfere with the TGF-β/Smad signaling pathway in a ligand-specific manner. Oncogene. 2003; 22:8212–8220.
Article9. Rhinn M, Dolle P. Retinoic acid signalling during development. Development. 2012; 139:843–858.
Article10. Barber T, Esteban-Pretel G, Marin MP, Timoneda J. Vitamin a deficiency and alterations in the extracellular matrix. Nutrients. 2014; 6:4984–5017.
Article11. Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 2004; 328:1–16.
Article12. Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011; 44:725–738.
Article13. Mahmood R, Flanders KC, Morriss-Kay GM. Interactions between retinoids and TGF beta s in mouse morphogenesis. Development. 1992; 115:67–74.
Article14. Redlich CA, Delisser HM, Elias JA. Retinoic acid inhibition of transforming growth factor-beta-induced collagen production by human lung fibroblasts. Am J Respir Cell Mol Biol. 1995; 12:287–295.
Article15. Torry DJ, Richards CD, Podor TJ, Gauldie J. Modulation of the anchorage-independent phenotype of human lung fibroblasts obtained from fibrotic tissue following culture with retinoid and corticosteroid. Exp Lung Res. 1996; 22:231–244.
Article16. Zhao Y, Geverd DA. Regulation of Smad3 expression in bleomycin-induced pulmonary fibrosis: a negative feedback loop of TGF-beta signaling. Biochem Biophys Res Commun. 2002; 294:319–323.17. Bonniaud P, Margetts PJ, Ask K, Flanders K, Gauldie J, Kolb M. TGF-beta and Smad3 signaling link inflammation to chronic fibrogenesis. J Immunol. 2005; 175:5390–5395.18. Tabata C, Kadokawa Y, Tabata R, Takahashi M, Okoshi K, Sakai Y, et al. All-trans-retinoic acid prevents radiation- or bleomycin-induced pulmonary fibrosis. Am J Respir Crit Care Med. 2006; 174:1352–1360.
Article19. Yu Z, Xing Y. All-trans retinoic acid inhibited chondrogenesis of mouse embryonic palate mesenchymal cells by down-regulation of TGF-beta/Smad signaling. Biochem Biophys Res Commun. 2006; 340:929–934.20. Falk LA, De Benedetti F, Lohrey N, Birchenall-Roberts MC, Ellingsworth LW, Faltynek CR, et al. Induction of transforming growth factor-beta 1 (TGF-beta 1), receptor expression and TGF-beta 1 protein production in retinoic acid-treated HL-60 cells: possible TGF-beta 1-mediated autocrine inhibition. Blood. 1991; 77:1248–1255.
Article21. Cao Z, Flanders KC, Bertolette D, Lyakh LA, Wurthner JU, Parks WT, et al. Levels of phospho-Smad2/3 are sensors of the interplay between effects of TGF-beta and retinoic acid on monocytic and granulocytic differentiation of HL-60 cells. Blood. 2003; 101:498–507.22. Taipale J, Matikainen S, Hurme M, Keski-Oja J. Induction of transforming growth factor beta 1 and its receptor expression during myeloid leukemia cell differentiation. Cell Growth Differ. 1994; 5:1309–1319.23. Furukawa F, Matsuzaki K, Mori S, Tahashi Y, Yoshida K, Sugano Y, et al. p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology. 2003; 38:879–889.
Article24. Harari S, Caminati A. IPF: new insight on pathogenesis and treatment. Allergy. 2010; 65:537–553.
Article25. Ryu JH, Moua T, Daniels CE, Hartman TE, Yi ES, Utz JP, et al. Idiopathic pulmonary fibrosis: evolving concepts. Mayo Clin Proc. 2014; 89:1130–1142.
Article26. Ross KR, Corey DA, Dunn JM, Kelley TJ. SMAD3 expression is regulated by mitogen-activated protein kinase kinase-1 in epithelial and smooth muscle cells. Cell Signal. 2007; 19:923–931.
Article27. Gui T, Sun Y, Shimokado A, Muragaki Y. The roles of mitogen-activated protein kinase pathways in TGF-beta-induced epithelial-mesenchymal transition. J Signal Transduct. 2012; 2012:289243.28. Engel ME, McDonnell MA, Law BK, Moses HL. Interdependent SMAD and JNK signaling in transforming growth factor-beta-mediated transcription. J Biol Chem. 1999; 274:37413–37420.29. Hartsough MT, Mulder KM. Transforming growth factor beta activation of p44mapk in proliferating cultures of epithelial cells. J Biol Chem. 1995; 270:7117–7124.30. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009; 139:871–890.
Article31. King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011; 378:1949–1961.
Article32. Shi K, Jiang J, Ma T, Xie J, Duan L, Chen R, et al. Pathogenesis pathways of idiopathic pulmonary fibrosis in bleomycin-induced lung injury model in mice. Respir Physiol Neurobiol. 2014; 190:113–117.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 4-O-Methylhonokiol Protects HaCaT Cells from TGF-β1-Induced Cell Cycle Arrest by Regulating Canonical and Non-Canonical Pathways of TGF-β Signaling
- Transforming Growth Factor β Receptor Type I Inhibitor, Galunisertib, Has No Beneficial Effects on Aneurysmal Pathological Changes in Marfan Mice
- Apolipoprotein A1 Inhibits TGF-β1-Induced Epithelial-to-Mesenchymal Transition of Alveolar Epithelial Cells
- Simvastatin inhibits sphingosylphosphorylcholine-induced differentiation of human mesenchymal stem cells into smooth muscle cells
- Relaxin Modulates the Expression of MMPs and TIMPs in Fibroblasts of Patients with Carpal Tunnel Syndrome