Yonsei Med J.
2017 Mar;58(2):415-422. 10.3349/ymj.2017.58.2.415.
Relaxin Modulates the Expression of MMPs and TIMPs in Fibroblasts of Patients with Carpal Tunnel Syndrome
- Affiliations
-
- 1BK21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Orthopedic Surgery, Yonsei University College of Medicine, Seoul, Korea. yrchoi@yuhs.ac
- KMID: 2427132
- DOI: http://doi.org/10.3349/ymj.2017.58.2.415
Abstract
- PURPOSE
The aim of this study was to investigate the anti-fibrotic effect of relaxin in subsynovial fibroblasts activated by transforming growth factor beta (TGF-β).
MATERIALS AND METHODS
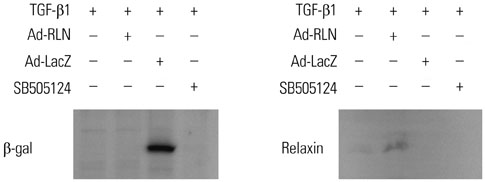
To test the anti-fibrotic effect of an adenovirus-relaxin construct (Ad-RLN) on subsynovial fibroblasts in vitro, cells from subsynovial connective tissue of patients with carpal tunnel syndrome were activated with TGF-β1 and exposed to Ad-RLN (as a therapeutic gene) or adenovirus-lacZ construct (as a marker gene) for four hours. Subsynovial fibroblast cultures without adenoviral exposure served as controls.
RESULTS
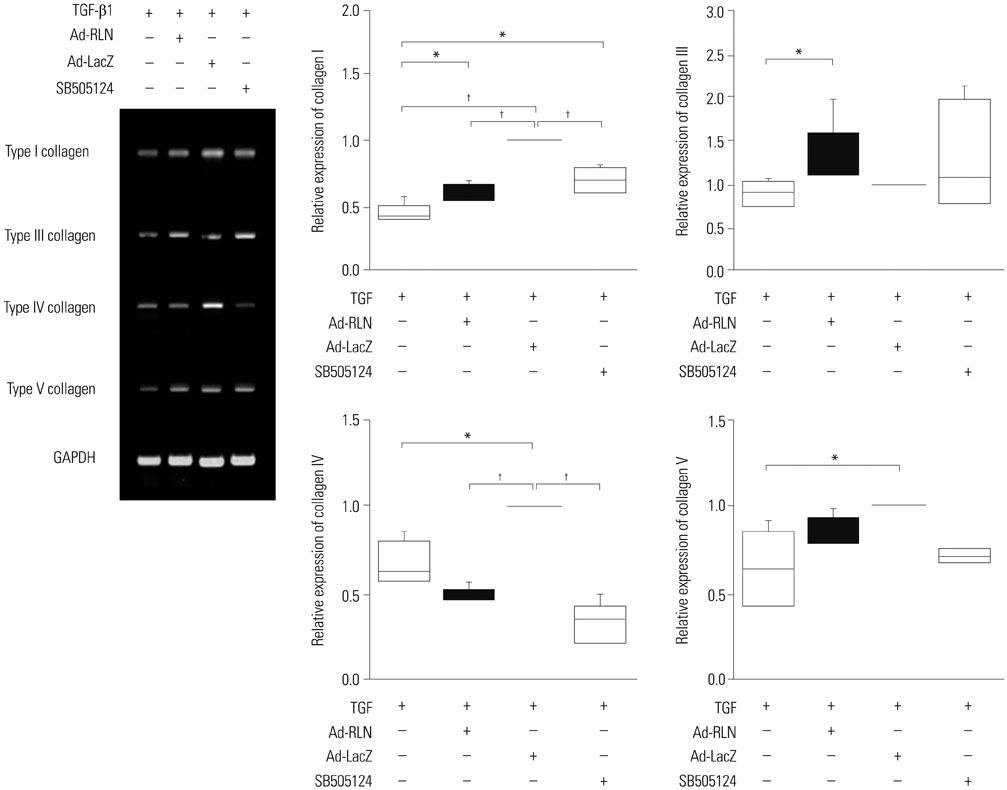
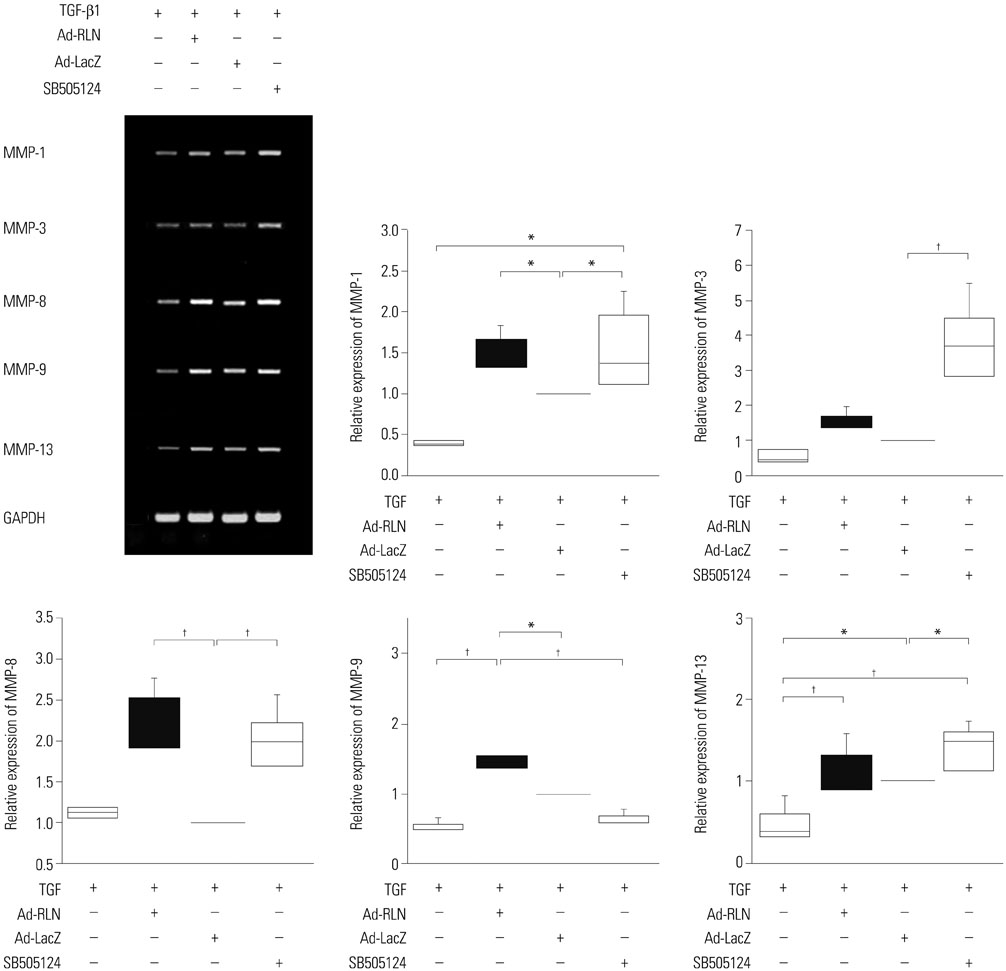
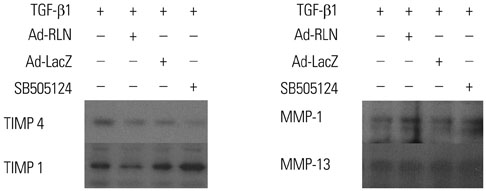
We observed induction of gene expressions of collagen I, III and IV, as well as the abatement of alpha-smooth muscle actin (a-SMA) synthesis, Smad2 phosphorylation, and fibronectin at the protein level, in comparison to controls. In addition, protein expressions of matrix metalloproteinase (MMP) I was significantly induced, whereas the protein expressions of tissue inhibitor of metalloproteinases (TIMP) I and IV were reduced due to relaxin expression.
CONCLUSION
RLN prevents excessive synthesis of extracellular matrix by reducing the expressions of its components, such as fibronectin, a-SMA, and phosphorylated Smad2, by increasing the expression of MMPs; and by decreasing the expression of TIMPs.
Keyword
MeSH Terms
-
Carpal Tunnel Syndrome/*metabolism
Cells, Cultured
Collagen Type I/metabolism
Collagen Type III/metabolism
Collagen Type IV/metabolism
Extracellular Matrix/metabolism
Fibroblasts/drug effects/*metabolism
Fibronectins/metabolism
Humans
Matrix Metalloproteinases/*metabolism
Relaxin/*pharmacology
Smad2 Protein/metabolism
Tissue Inhibitor of Metalloproteinases/*metabolism
Transforming Growth Factor beta/metabolism
Transforming Growth Factor beta1/pharmacology
Collagen Type I
Collagen Type III
Collagen Type IV
Fibronectins
Smad2 Protein
Tissue Inhibitor of Metalloproteinases
Transforming Growth Factor beta
Transforming Growth Factor beta1
Relaxin
Matrix Metalloproteinases
Figure
Reference
-
1. Armstrong TJ, Castelli WA, Evans FG, Diaz-Perez R. Some histological changes in carpal tunnel contents and their biomechanical implications. J Occup Med. 1984; 26:197–201.2. Ettema AM, Amadio PC, Zhao C, Wold LE, An KN. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg Am. 2004; 86-A:1458–1466.
Article3. Nakamichi K, Tachibana S. Histology of the transverse carpal ligament and flexor tenosynovium in idiopathic carpal tunnel syndrome. J Hand Surg Am. 1998; 23:1015–1024.
Article4. Neal NC, McManners J, Stirling GA. Pathology of the flexor tendon sheath in the spontaneous carpal tunnel syndrome. J Hand Surg Br. 1987; 12:229–232.
Article5. Oh J, Zhao C, Amadio PC, An KN, Zobitz ME, Wold LE. Immunolocalization of collagen types in the subsynovial connective tissue within the carpal tunnel in humans. J Orthop Res. 2005; 23:1226–1231.
Article6. Ettema AM, Amadio PC, Zhao C, Wold LE, O'Byrne MM, Moran SL, et al. Changes in the functional structure of the tenosynovium in idiopathic carpal tunnel syndrome: a scanning electron microscope study. Plast Reconstr Surg. 2006; 118:1413–1422.
Article7. Schuind F, Ventura M, Pasteels JL. Idiopathic carpal tunnel syndrome: histologic study of flexor tendon synovium. J Hand Surg Am. 1990; 15:497–503.
Article8. Ettema AM, Zhao C, An KN, Amadio PC. Comparative anatomy of the subsynovial connective tissue in the carpal tunnel of the rat, rabbit, dog, baboon, and human. Hand (N Y). 2006; 1:78–84.
Article9. Jinrok O, Zhao C, Amadio PC, An KN, Zobitz ME, Wold LE. Vascular pathologic changes in the flexor tenosynovium (subsynovial connective tissue) in idiopathic carpal tunnel syndrome. J Orthop Res. 2004; 22:1310–1315.
Article10. Zhao C, Ettema AM, Berglund LJ, An KN, Amadio PC. Gliding resistance of flexor tendon associated with carpal tunnel pressure: a biomechanical cadaver study. J Orthop Res. 2011; 29:58–61.
Article11. Keir PJ, Rempel DM. Pathomechanics of peripheral nerve loading. Evidence in carpal tunnel syndrome. J Hand Ther. 2005; 18:259–269.12. Ettema AM, An KN, Zhao C, O'Byrne MM, Amadio PC. Flexor tendon and synovial gliding during simultaneous and single digit flexion in idiopathic carpal tunnel syndrome. J Biomech. 2008; 41:292–298.
Article13. Yamaguchi T, Osamura N, Zhao C, Zobitz ME, An KN, Amadio PC. The mechanical properties of the rabbit carpal tunnel subsynovial connective tissue. J Biomech. 2008; 41:3519–3522.
Article14. Osamura N, Zhao C, Zobitz ME, An KN, Amadio PC. Evaluation of the material properties of the subsynovial connective tissue in carpal tunnel syndrome. Clin Biomech (Bristol, Avon). 2007; 22:999–1003.
Article15. Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002; 118:211–215.
Article16. Gauldie J, Bonniaud P, Sime P, Ask K, Kolb M. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem Soc Trans. 2007; 35(Pt 4):661–664.17. Mauviel A. Transforming growth factor-beta: a key mediator of fibrosis. Methods Mol Med. 2005; 117:69–80.18. Schwabe C, Büllesbach EE. Relaxin, the relaxin-like factor and their receptors. Adv Exp Med Biol. 2007; 612:14–25.
Article19. Formigli L, Francini F, Nistri S, Margheri M, Luciani G, Naro F, et al. Skeletal myoblasts overexpressing relaxin improve differentiation and communication of primary murine cardiomyocyte cell cultures. J Mol Cell Cardiol. 2009; 47:335–345.
Article20. Kang YM, Choi YR, Yun CO, Park JO, Suk KS, Kim HS, et al. Down-regulation of collagen synthesis and matrix metalloproteinase expression in myofibroblasts from Dupuytren nodule using adenovirus-mediated relaxin gene therapy. J Orthop Res. 2014; 32:515–523.
Article21. Kim JH, Lee YS, Kim H, Huang JH, Yoon AR, Yun CO. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J Natl Cancer Inst. 2006; 98:1482–1493.
Article22. Yoshii Y, Zhao C, Schmelzer JD, Low PA, An KN, Amadio PC. The effects of hypertonic dextrose injection on connective tissue and nerve conduction through the rabbit carpal tunnel. Arch Phys Med Rehabil. 2009; 90:333–339.
Article23. Tekin F, Sürmeli M, S¸ims¸ek H, Ceran C, Tezcan S, Taner ÖF, et al. Comparison of the histopathological findings of patients with diabetic and idiopathic carpal tunnel syndrome. Int Orthop. 2015; 39:2395–2401.
Article24. Yeşil M, Bacakaoğlu AK, Dogğan M. Are myofibroblasts activated in idiopathic carpal tunnel syndrome? an immunohistochemical study. Eklem Hastalik Cerrahisi. 2014; 25:133–140.
Article25. Tat J, Wilson KE, Keir PJ. Pathological changes in the subsynovial connective tissue increase with self-reported carpal tunnel syndrome symptoms. Clin Biomech (Bristol, Avon). 2015; 30:360–365.
Article26. Hinz B. Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep. 2009; 11:120–126.
Article27. Chikenji T, Gingery A, Zhao C, Passe SM, Ozasa Y, Larson D, et al. Transforming growth factor-β (TGF-β) expression is increased in the subsynovial connective tissues of patients with idiopathic carpal tunnel syndrome. J Orthop Res. 2014; 32:116–122.
Article28. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008; 16:585–601.29. Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994; 331:1286–1292.30. Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004; 18:816–827.31. Gingery A, Yang TH, Passe SM, An KN, Zhao C, Amadio PC. TGF-β signaling regulates fibrotic expression and activity in carpal tunnel syndrome. J Orthop Res. 2014; 32:1444–1450.
Article32. Millan FA, Denhez F, Kondaiah P, Akhurst RJ. Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo. Development. 1991; 111:131–143.
Article33. Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-beta /Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001; 276:17058–17062.
Article34. Ucan H, Yagci I, Yilmaz L, Yagmurlu F, Keskin D, Bodur H. Comparison of splinting, splinting plus local steroid injection and open carpal tunnel release outcomes in idiopathic carpal tunnel syndrome. Rheumatol Int. 2006; 27:45–51.
Article35. Samuel CS, Lin F, Hossain MA, Zhao C, Ferraro T, Bathgate RA, et al. Improved chemical synthesis and demonstration of the relaxin receptor binding affinity and biological activity of mouse relaxin. Biochemistry. 2007; 46:5374–5381.
Article36. van der Westhuizen ET, Halls ML, Samuel CS, Bathgate RA, Unemori EN, Sutton SW, et al. Relaxin family peptide receptors--from orphans to therapeutic targets. Drug Discov Today. 2008; 13:640–651.37. Hisaw FL. Experimental relaxation of the pubic ligament of the guinea pig. Proc Soc Exp Biol Med. 1926; 23:661–663.
Article38. Masterson R, Hewitson TD, Kelynack K, Martic M, Parry L, Bathgate R, et al. Relaxin down-regulates renal fibroblast function and promotes matrix remodelling in vitro. Nephrol Dial Transplant. 2004; 19:544–552.
Article39. Unemori EN, Pickford LB, Salles AL, Piercy CE, Grove BH, Erikson ME, et al. Relaxin induces an extracellular matrix-degrading phenotype in human lung fibroblasts in vitro and inhibits lung fibrosis in a murine model in vivo. J Clin Invest. 1996; 98:2739–2745.
Article40. Unemori EN, Amento EP. Relaxin modulates synthesis and secretion of procollagenase and collagen by human dermal fibroblasts. J Biol Chem. 1990; 265:10681–10685.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ultrasound-Guided Nerve Hydrodissection for Carpal Tunnel Syndrome
- Carpal Tunnel Syndrome in Children with Hypogammaglobulinemia: Case Report
- The Current Concepts for the Pathophysiology of Idiopathic Carpal Tunnel Syndrome
- The Carpal compression Test for Diagnosing Carpal Tunnel Syndrome
- Usefulness of Ultrasound for Carpal Tunnel Syndrome Proven in Meta-Analysis Studies