Korean J Physiol Pharmacol.
2019 Mar;23(2):121-130. 10.4196/kjpp.2019.23.2.121.
Neuroprotective mechanisms of dieckol against glutamate toxicity through reactive oxygen species scavenging and nuclear factor-like 2/heme oxygenase-1 pathway
- Affiliations
-
- 1Department of Physiology, Jeju National University School of Medicine, Jeju 63243, Korea. syeun@jejunu.ac.kr
- 2Department of Neurosurgery, Jeju National University School of Medicine, Jeju 63243, Korea.
- 3Division of Hematology-Oncology, Department of Internal Medicine, Jeju National University School of Medicine, Jeju 63243, Korea.
- 4Neurology 1, The Second Affiliated Hospital of Xinxiang Medical University, Henan 453002, China.
- 5BotaMedi Inc., Jeju 63309, Korea.
- 6Center for Cognition and Sociality, Institute for Basic Science (IBS), KAIST, Daejeon 34126, Korea.
- 7University of Science and Technology, Daejeon 34113, Korea.
- 8Institute of Medical Science, Jeju National University, Jeju 63243, Korea.
- KMID: 2438086
- DOI: http://doi.org/10.4196/kjpp.2019.23.2.121
Abstract
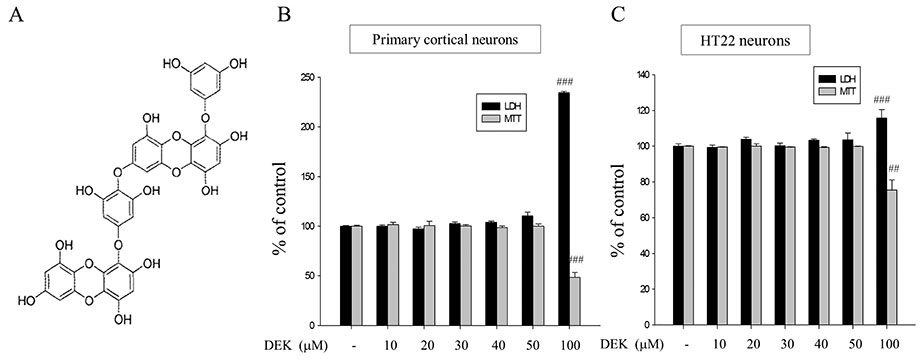
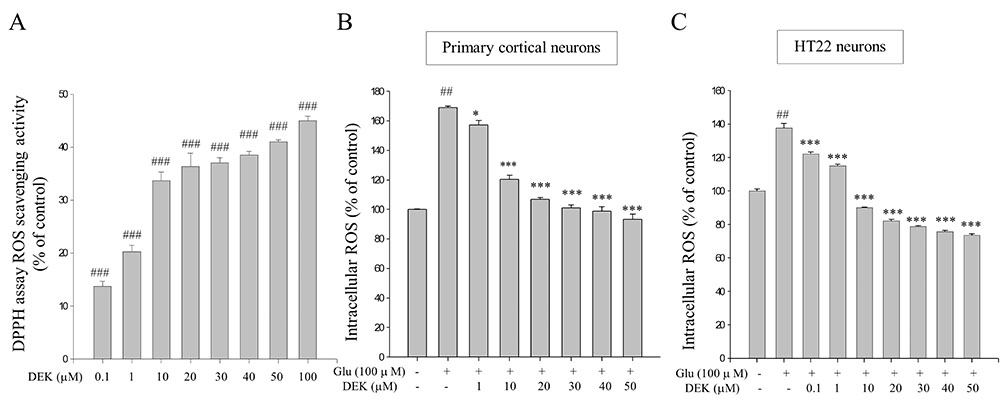
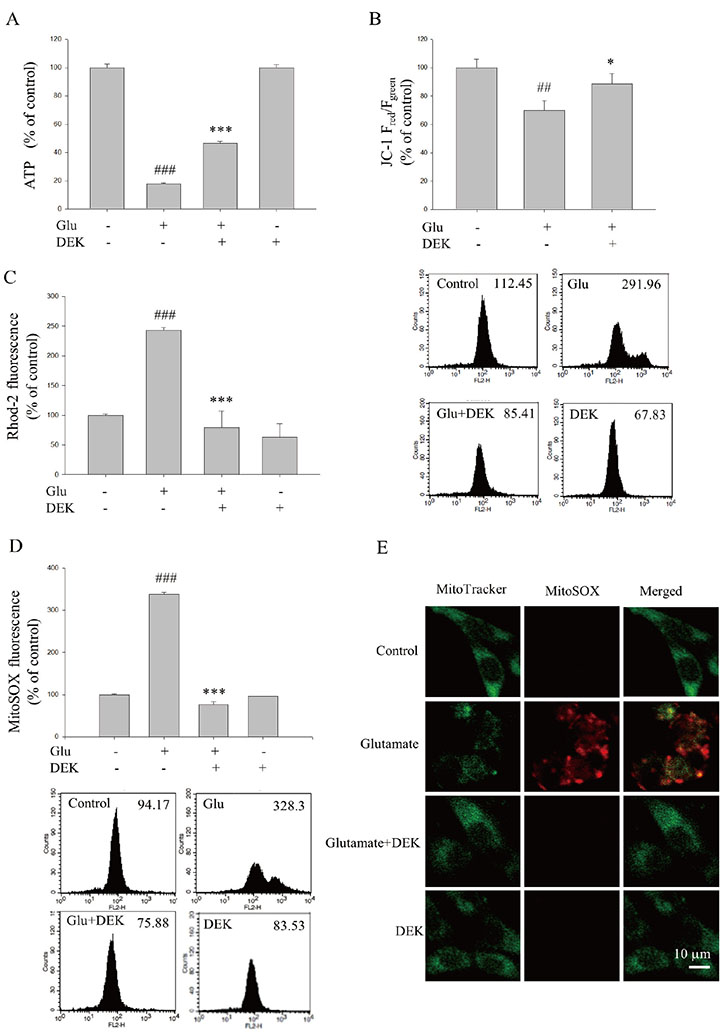
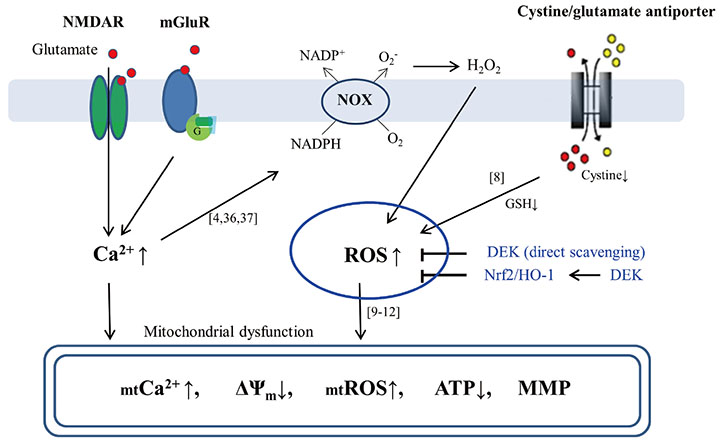
- Glutamate toxicity-mediated mitochondrial dysfunction and neuronal cell death are involved in the pathogenesis of several neurodegenerative diseases as well as acute brain ischemia/stroke. In this study, we investigated the neuroprotective mechanism of dieckol (DEK), one of the phlorotannins isolated from the marine brown alga Ecklonia cava, against glutamate toxicity. Primary cortical neurons (100 µM, 24 h) and HT22 neurons (5 mM, 12 h) were stimulated with glutamate to induce glutamate toxic condition. The results demonstrated that DEK treatment significantly increased cell viability in a dose-dependent manner (1-50 µM) and recovered morphological deterioration in glutamate-stimulated neurons. In addition, DEK strongly attenuated intracellular reactive oxygen species (ROS) levels, mitochondrial overload of Ca²âº and ROS, mitochondrial membrane potential (ΔΨ(m)) disruption, adenine triphosphate depletion. DEK showed free radical scavenging activity in the cell-free system. Furthermore, DEK enhanced protein expression of heme oxygenase-1 (HO-1), an important anti-oxidant enzyme, via the nuclear translocation of nuclear factor-like 2 (Nrf2). Taken together, we conclude that DEK exerts neuroprotective activities against glutamate toxicity through its direct free radical scavenging property and the Nrf-2/HO-1 pathway activation.
Keyword
MeSH Terms
Figure
Reference
-
1. Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988; 1:623–634.
Article2. Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003; 4:552–565.
Article3. Duchen MR. Mitochondria, calcium-dependent neuronal death and neurodegenerative disease. Pflugers Arch. 2012; 464:111–121.
Article4. Ha JS, Lee JE, Lee JR, Lee CS, Maeng JS, Bae YS, Kwon KS, Park SS. Nox4-dependent H2O2 production contributes to chronic glutamate toxicity in primary cortical neurons. Exp Cell Res. 2010; 316:1651–1661.5. Peng TI, Jou MJ. Oxidative stress caused by mitochondrial calcium overload. Ann N Y Acad Sci. 2010; 1201:183–188.
Article6. Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron. 1989; 2:1547–1558.
Article7. Tobaben S, Grohm J, Seiler A, Conrad M, Plesnila N, Culmsee C. Bid-mediated mitochondrial damage is a key mechanism in glutamate-induced oxidative stress and AIF-dependent cell death in immortalized HT-22 hippocampal neurons. Cell Death Differ. 2011; 18:282–292.
Article8. Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron. 1997; 19:453–463.
Article9. Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000; 290:1761–1765.
Article10. Pei Y, Xing D, Gao X, Liu L, Chen T. Real-time monitoring full length bid interacting with Bax during TNF-alpha-induced apoptosis. Apoptosis. 2007; 12:1681–1690.11. Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007; 87:99–163.
Article12. Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999; 397:441–446.
Article13. Susin SA, Daugas E, Ravagnan L, Samejima K, Zamzami N, Loeffler M, Costantini P, Ferri KF, Irinopoulou T, Prévost MC, Brothers G, Mak TW, Penninger J, Earnshaw WC, Kroemer G. Two distinct pathways leading to nuclear apoptosis. J Exp Med. 2000; 192:571–580.
Article14. Cregan SP, Fortin A, MacLaurin JG, Callaghan SM, Cecconi F, Yu SW, Dawson TM, Dawson VL, Park DS, Kroemer G, Slack RS. Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death. J Cell Biol. 2002; 158:507–517.
Article15. Slemmer JE, Zhu C, Landshamer S, Trabold R, Grohm J, Ardeshiri A, Wagner E, Sweeney MI, Blomgren K, Culmsee C, Weber JT, Plesnila N. Causal role of apoptosis-inducing factor for neuronal cell death following traumatic brain injury. Am J Pathol. 2008; 173:1795–1805.
Article16. Valencia A, Sapp E, Kimm JS, McClory H, Reeves PB, Alexander J, Ansong KA, Masso N, Frosch MP, Kegel KB, Li X, DiFiglia M. Elevated NADPH oxidase activity contributes to oxidative stress and cell death in Huntington's disease. Hum Mol Genet. 2013; 22:1112–1131.
Article17. Cristóvão AC, Guhathakurta S, Bok E, Je G, Yoo SD, Choi DH, Kim YS. NADPH oxidase 1 mediates α-synucleinopathy in Parkinson's disease. J Neurosci. 2012; 32:14465–14477.18. Choi BY, Jang BG, Kim JH, Lee BE, Sohn M, Song HK, Suh SW. Prevention of traumatic brain injury-induced neuronal death by inhibition of NADPH oxidase activation. Brain Res. 2012; 1481:49–58.
Article19. Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999; 274:26071–26078.
Article20. Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003; 43:233–260.
Article21. Nguyen T, Sherratt PJ, Nioi P, Yang CS, Pickett CB. Nrf2 controls constitutive and inducible expression of ARE-driven genes through a dynamic pathway involving nucleocytoplasmic shuttling by Keap1. J Biol Chem. 2005; 280:32485–32492.
Article22. Kim AR, Shin TS, Lee MS, Park JY, Park KE, Yoon NY, Kim JS, Choi JS, Jang BC, Byun DS, Park NK, Kim HR. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J Agric Food Chem. 2009; 57:3483–3489.23. Koirala P, Jung HA, Choi JS. Recent advances in pharmacological research on Ecklonia species: a review. Arch Pharm Res. 2017; 40:981–1005.
Article24. Myung CS, Shin HC, Bao HY, Yeo SJ, Lee BH, Kang JS. Improvement of memory by dieckol and phlorofucofuroeckol in ethanol-treated mice: possible involvement of the inhibition of acetylcholinesterase. Arch Pharm Res. 2005; 28:691–698.
Article25. Cui Y, Park JY, Wu J, Lee JH, Yang YS, Kang MS, Jung SC, Park JM, Yoo ES, Kim SH, Ahn Jo S, Suk K, Eun SY. Dieckol attenuates microglia-mediated neuronal cell death via ERK, Akt and NADPH oxidase-mediated pathways. Korean J Physiol Pharmacol. 2015; 19:219–228.
Article26. Li Y, Qian ZJ, Ryu B, Lee SH, Kim MM, Kim SK. Chemical components and its antioxidant properties in vitro: an edible marine brown alga, Ecklonia cava. Bioorg Med Chem. 2009; 17:1963–1973.
Article27. Breyer A, Elstner M, Gillessen T, Weiser D, Elstner E. Glutamate-induced cell death in neuronal HT22 cells is attenuated by extracts from St. John's wort (Hypericum perforatum L.). Phytomedicine. 2007; 14:250–255.
Article28. Cui Y, Wu J, Jung SC, Park DB, Maeng YH, Hong JY, Kim SJ, Lee SR, Kim SJ, Kim SJ, Eun SY. Anti-neuroinflammatory activity of nobiletin on suppression of microglial activation. Biol Pharm Bull. 2010; 33:1814–1821.
Article29. Wu JJ, Cui Y, Yang YS, Jung SC, Hyun JW, Maeng YH, Park DB, Lee SR, Kim SJ, Eun SY. Mild mitochondrial depolarization is involved in a neuroprotective mechanism of Citrus sunki peel extract. Phytother Res. 2013; 27:564–571.30. Lee JH, Amarsanaa K, Wu J, Jeon SC, Cui Y, Jung SC, Park DB, Kim SJ, Han SH, Kim HW, Rhyu IJ, Eun SY. Nobiletin attenuates neurotoxic mitochondrial calcium overload through K+ influx and ΔΨm across mitochondrial inner membrane. Korean J Physiol Pharmacol. 2018; 22:311–319.31. Zhang Y, Bhavnani BR. Glutamate-induced apoptosis in neuronal cells is mediated via caspase-dependent and independent mechanisms involving calpain and caspase-3 proteases as well as apoptosis inducing factor (AIF) and this process is inhibited by equine estrogens. BMC Neurosci. 2006; 7:49.32. Noh HS, Hah YS, Nilufar R, Han J, Bong JH, Kang SS, Cho GJ, Choi WS. Acetoacetate protects neuronal cells from oxidative glutamate toxicity. J Neurosci Res. 2006; 83:702–709.
Article33. Kang TH, Bae KH, Yu MJ, Kim WK, Hwang HR, Jung H, Lee PY, Kang S, Yoon TS, Park SG, Ryu SE, Lee SC. Phosphoproteomic analysis of neuronal cell death by glutamate-induced oxidative stress. Proteomics. 2007; 7:2624–2635.
Article34. Azadmanesh J, Borgstahl GEO. A review of the catalytic mechanism of human manganese superoxide dismutase. Antioxidants (Basel). 2018; 7:pii: E25.
Article35. Chen C, Li L, Zhou HJ, Min W. The role of NOX4 and TRX2 in angiogenesis and their potential cross-talk. Antioxidants (Basel). 2017; 6:pii: E42.
Article36. Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009; 12:857–863.
Article37. Brennan-Minnella AM, Shen Y, El-Benna J, Swanson RA. Phosphoinositide 3-kinase couples NMDA receptors to superoxide release in excitotoxic neuronal death. Cell Death Dis. 2013; 4:e580.
Article38. Kwak JH, He Y, Yoon B, Koo S, Yang Z, Kang EJ, Lee BH, Han SY, Yoo YC, Lee KB, Kim JS. Synthesis of rhodamine-labelled dieckol: its unique intracellular localization and potent anti-inflammatory activity. Chem Commun (Camb). 2014; 50:13045–13048.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fraxetin Induces Heme Oxygenase-1 Expression by Activation of Akt/Nrf2 or AMP-activated Protein Kinase α/Nrf2 Pathway in HaCaT Cells
- Cytoprotective Effects of Serum Hormone Deprivation against Glutamate Toxicity in HT22 Mouse Hippocampal Cells
- Downregulation of Reactive Oxygen Species in Apoptosis
- SiO2 Nanoparticles Induced Cytotoxicity by Oxidative Stress in Human Bronchial Epithelial Cell, Beas-2B
- Neuroprotective Effect of β-Lapachone against Glutamate-Induced Injury in HT22 Cells