Nutr Res Pract.
2010 Feb;4(1):3-10.
Desalinated underground seawater of Jeju Island (Korea) improves lipid metabolism in mice fed diets containing high fat and increases antioxidant potential in t-BHP treated HepG2 cells
- Affiliations
-
- 1Animal Model Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), 111 Gwahangno, Yuseong-gu, Daejeon 305-806, Korea.
- 2Hi-Tech Industry Development Institute, Jeju 690-121, Korea.
- 3College of Pharmacy, Chosun University, Gwangju 501-759, Korea.
- 4Marine and Environmental Research Institute, Jeju National University, Jeju 695-814, Korea. leemri@cheju.ac.kr
Abstract
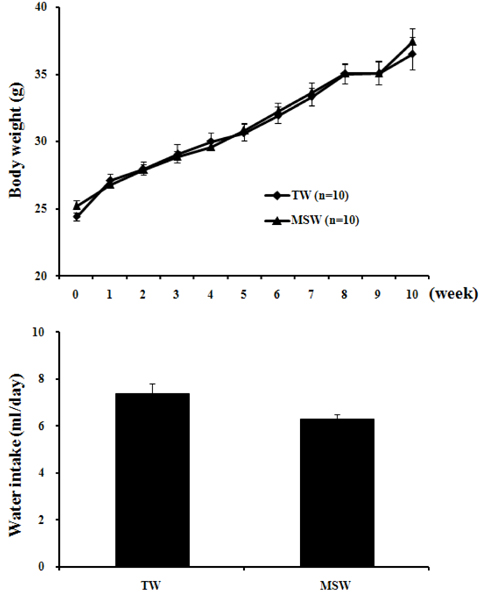
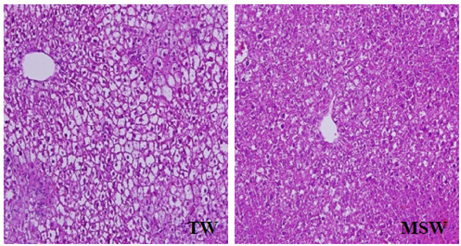
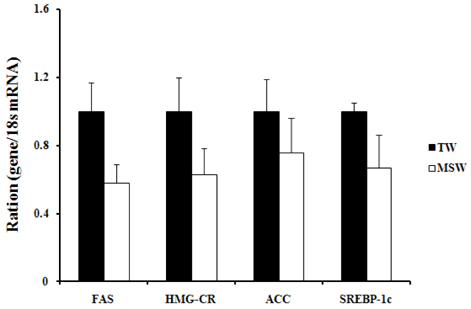
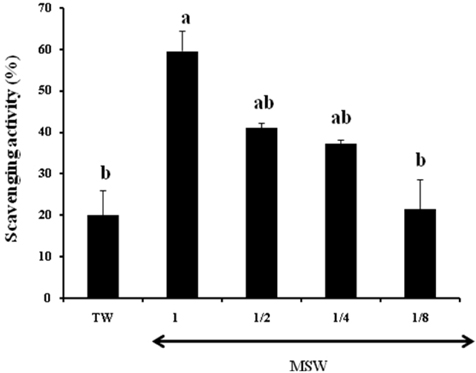
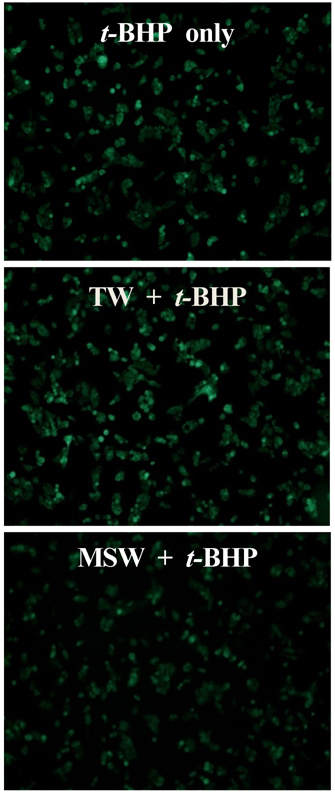
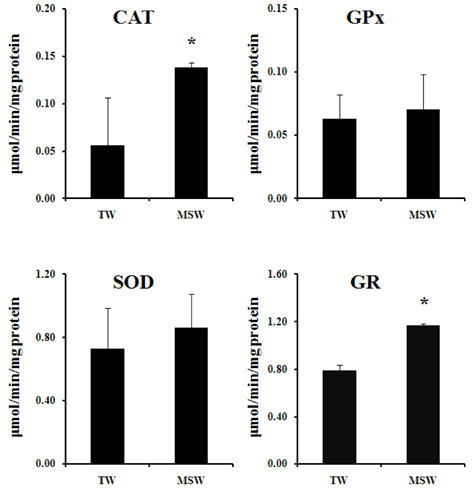
- This study was performed to investigate the effect of desalinated underground seawater (named as 'magma seawater', MSW) of Jeju Island in Korea on lipid metabolism and antioxidant activity. MSW was collected from underground of Han-Dong in Jeju Island, and freely given to high fat diet (HFD)-fed C57BL/6 mice for 10 weeks. Although there were no significant differences in the body weight changes and plasma lipid levels, hepatic triglyceride levels were significantly lower in the MSW group than in the normal tap water (TW)-drunken control group. Furthermore, the activity of fatty acid synthase (FAS) was significantly decreased and carnitine palmitoyltransferase (CPT) activity was increased in MSW group compared to TW group. Similarly, real-time PCR analysis revealed that mRNA expressions of lipogenic genes were lowered in MSW groups compared to the control group. In a morphometric observation on the liver tissue, accumulation of fats was remarkably reduced in MSW group. Meanwhile, in vitro assay, free radical scavenging activity measured by using diphenylpicrylhydrazyl (DPPH) was increased in MSW group. The 2'-7'-dichlorofluorescein diacetate (DCF-DA) staining followed with fluorescent microscopy showed a low intensity of fluorescence in MSW-treated HepG2 cells, compared to TW-treated HepG2 cells, which indicated that the production of reactive oxygen species by tert-butyl hydroperoxide (t-BHP) in HepG2 cells was decreased by MSW treatment. The antioxidant effect of MSW on t-BHP-induced oxidative stress in HepG2 cells was supported by the increased activities of intracellular antioxidant enzymes such as catalase and glutathione reductase. From these results, we speculate that MSW has an inhibitory effect on lipogenesis in liver and might play a protective role against cell damage by t-BHP-induced oxidative stress.
MeSH Terms
-
Animals
Antioxidants
Body Weight Changes
Carnitine O-Palmitoyltransferase
Catalase
Diet
Diet, High-Fat
Fats
Fatty Acid Synthetase Complex
Fluorescence
Glutathione Reductase
Hep G2 Cells
Korea
Lipid Metabolism
Lipogenesis
Liver
Mice
Microscopy
Oxidative Stress
Plasma
Reactive Oxygen Species
Real-Time Polymerase Chain Reaction
RNA, Messenger
Seawater
tert-Butylhydroperoxide
Water
Antioxidants
Carnitine O-Palmitoyltransferase
Catalase
Fats
Fatty Acid Synthetase Complex
Glutathione Reductase
RNA, Messenger
Reactive Oxygen Species
Water
tert-Butylhydroperoxide
Figure
Reference
-
1. Hataguchi Y, Tai H, Nakajima H, Kimata H. Drinking deep-seawater restores mineral imbalance in atopic eczema/dermatitis syndrome. Eur J Clin Nutr. 2005. 59:1093–1096.
Article2. Nakasone T, Akeda S. The application of deep sea water in Japan. UJNR Technical Report. 1999. 28:69–75.3. Aoyama M, Hirose K, Miyao T, Igarashi Y. Low level 137Cs measurements in deep seawater samples. Appl Radiat Isot. 2000. 53:159–162.
Article4. Katsuda S, Nakagawa K, Miyake M, Yamasaki M, Katahira K, Mohri M, Shimizu T, Hazama A. Deep-seawater improves cardiovascular hemodynamics in Kurosawa and Kusanagi-Hypercholesterolemic (KHC) rabbits. Biol Pharm Bull. 2008. 31:38–44.
Article5. Miyamura M, Yoshioka S, Hamada A, Takuma D, Yokota J, Kusunose M, Kyotani S, Kawakita H, Odani K, Tsutsu Y, Nishioka Y. Difference between deep seawater and surface seawater in the preventive effect of atherosclerosis. Biol Pharm Bull. 2004. 27:1784–1787.
Article6. Tsuchiya Y, Watanabe A, Fujisawa N, Kaneko T, Ishizu T, Fujimoto T, Nakamura K, Yamamoto M. Effects of Desalted Deep Seawater on Hematologic and Blood Chemical Values in Mice. Tohoku J Exp Med. 2004. 203:175–182.
Article7. Yoshioka S, Hamada A, Cui T, Yokota J, Yamamoto S, Kusunose M, Miyamura M, Kyotani S, Kaneda R, Tsutsui Y, Odani K, Odani I, Nishioka Y. Pharmacological activity of deep-sea water: examination of hyperlipemia prevention and medical treatment effect. Biol Pharm Bull. 2003. 26:1552–1559.
Article8. Hwang HS, Kim SH, Yoo YG, Chu YS, Shon YH, Nam KS, Yun JW. Inhibitory effect of deep sea water on differentiation of 3T3-L1 adipocytes. Mar Biotechnol (NY). 2008. 11:161–168.
Article9. Bloomgarden ZT. Magnesium deficiency, atherosclerosis, and health care. Diabetes Care. 1995. 18:1623–1627.10. Robertson DS. Magnesium or calcium hypophosphite could be a treatment for obesity in humans. Med Hypotheses. 2006. 66:439–440.
Article11. Rude RK. Magnesium deficiency and diabetes mellitus. Causes and effects. Postgrad Med. 1992. 92:217–219. 222–224.12. Huang QL, Jin Y, Zhang LN, Cheung PCK, Kennedy JF. Structure, molecular size and antitumor activities of polysaccharides from Poria cocos mycelia produced in fermenter. Carbohydr Polym. 2007. 70:324–333.
Article13. Ibrahim W, Lee US, Yeh CC, Szabo J, Bruckner G, Chow CK. Oxidative stress and antioxidant status in mouse liver: effects of dietary lipid, vitamin E and iron. J Nutr. 1997. 127:1401–1406.
Article14. Mursu J, Voutilainen S, Nurmi T, Rissanen TH, Virtanen JK, Kaikkonen J, Nyyssönen K, Salonen JT. Dark Chocolate Consumption Increases HDL Cholesterol Concentration and Chocolate Fatty Acids May Inhibit Lipid Peroxidation in Healthy Humans. Free Radic Biol Med. 2004. 37:1351–1359.
Article15. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959. 37:911–917.
Article16. Hulcher PH, Oleson WH. Simplified spectrophotometric assay for microsomal 3-hydroxy-3-methylglutaryl CoA reductase by measurement of coenzyme A. J Lipid Res. 1973. 14:625–631.
Article17. Nepokroeff CM, Lakshmanan MR, Porter JW. Fatty-acid synthase from rat liver. Meth Enzymol. 1975. 35:37–44.18. Markwell MA, McGroarty EJ, Bieber LL, Tolbert NE. The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. A new peroxisomal enzyme. J Biol Chem. 1973. 248:3426–3432.
Article19. Yoshikawa T, Naito Y, Kondo M. Hiramatsu M, Yoshikawa T, Inoue M, editors. Food and diseases 2. Free radicals and diseases. 1997. New York: 11–19.20. Yamaguchi T, Takamura H, Matoba T, Terao J. HPLC method for evaluation of the free radical-scavenging activity of food by using 1,1-diphenyl-2-picrylhydrazyl. Biosci Biotechnol Biochem. 1998. 62:1201–1204.
Article21. Aebi H. Bergmeyer HU, editor. Catalase. Methods of enzymatic analysis. 1974. . New York: Academic press;673–684.
Article22. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974. 47:469–474.
Article23. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967. 70:158–169.24. Pinto RE, Bartley W. The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem J. 1969. 112:109–115.
Article25. Naik GH, Priyadarsini KI, Satav JG, Banavalikar MM, Sohoni DP, Biyani MK, Mohan H. Comparative antioxidant activity of individual herbal components used in Ayurvedic medicine. Phytochemistry. 2003. 63:97–104.
Article26. Latasa MJ, Griffin MJ, Noon YS, Kang CH, Sul HS. Occupancy and function of the 150 sterol regulatory element and -65 E-box in nutritional regulation of the fatty acid synthase gene in living animals. Mol Cell Biol. 2003. 23:5896–5907.
Article27. McGarry JD, Brown NF. The mitochondrial carnitine palmitoy-ltransferase system. From concept to molecular analysis. Eur J Biochem. 1997. 244:1–14.
Article28. Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002. 109:1125–1131.
Article29. Paulauskis JD, Sul HS. Hormonal regulation of mouse fatty acid synthase gene transcription in liver. J Biol Chem. 1989. 264:574–577.
Article30. Hwa JJ, Zollman S, Warden CH, Taylor BA, Edwards PA, Fogelman AM, Lusis AJ. Genetic and dietary interactions in the regulation of HMG-CoA reductase gene expression. J Lipid Res. 1992. 33:711–725.
Article31. Iacono JM. Effect of varying the dietary level of calcium on plasma and tissue lipids of rabbits. J Nutr. 1974. 104:1165–1171.
Article32. Yacowitz H, Fleischman AI, Amsden RT, Bierenbaum ML. Effects of dietary calcium upon lipid metabolism in rats fed saturated or unsaturated fat. J Nutr. 1967. 92:389–392.
Article33. Lukaski HC, Nielsen FH. Dietary magnesium depletion affects metabolic responses during submaximal exercise in postmenopausal women. J Nutr. 2007. 132:930–935.
Article34. Garcia LA, Dejong SC, Martin SM, Smith RS, Buttner GR, Kerber RE. Magnesium reduces free radicals in an in vivo coronary occlusion-reperfusion model. J Am Coll Cardiol. 1998. 32:536–539.
Article35. Kumar BP, Shivakumar K. Depressed antioxidant defense in rat heart in experimental magnesium deficiency. Implications for the pathogenesis of myocardial lesions. Biol Trace Elem Res. 1997. 60:139–144.
Article36. Fagali N, Catalá A. Antioxidant activity of conjugated linoleic acid isomers, linoleic acid and its methyl ester determined by photoemission and DPPH techniques. Biophys Chem. 2008. 137:56–62.
Article37. Hirano R, Sasamoto W, Matsumoto A, Itakura H, Igarashi O, Kondo K. Antioxidant ability of various flavonoids against DPPH radicals and LDL oxidation. J Nutr Sci Vitaminol (Tokyo). 2001. 47:357–362.
Article38. Martín MA, Ramos S, Mateos R, Granado Serrano AB, Izquierdo-Pulido M, Bravo L, Goya L. Protection of human HepG2 cells against oxidative stress by cocoa phenolic extract. J Agric Food Chem. 2008. 56:7765–7772.
Article39. Sies H. Strategies of antioxidative defense. Eur J Biochem. 1993. 215:213–219.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Soyoligosaccharide on Lipid Metabolism in Rats Fed the High Fat or Low Fat Diet
- Isolation of Vibrio vulnificus from Seawater and Emerging Vibrio vulnificus Septicemia on Jeju Island
- Doxorubicin Attenuates Free Fatty Acid-Induced Lipid Accumulation via Stimulation of p53 in HepG2 Cells
- Effects of Liquid Culture of Coriolus Versicolor on Lipid Metabolism and Enzyme Activities in Rats Fed High Fat Diet
- Grape skin improves antioxidant capacity in rats fed a high fat diet