Ann Dermatol.
2018 Oct;30(5):566-574. 10.5021/ad.2018.30.5.566.
The Suppressive Effect of Butyrate and Bromopyruvate on Inflammatory Cytokine Production and Short Chain Fatty Acid Receptor Expression by Blood Mononuclear Cells in Patients with Behçet's Disease
- Affiliations
-
- 1Department of Microbiology, Ajou University School of Medicine, Suwon, Korea. sinsun@ajou.ac.kr
- 2Department of Biomedical Sciences, The Graduate School, Ajou University, Suwon, Korea.
- 3Department of Dermatology, Ajou University School of Medicine, Suwon, Korea. esl@ajou.ac.kr
- KMID: 2419747
- DOI: http://doi.org/10.5021/ad.2018.30.5.566
Abstract
- BACKGROUND
Controlling inflammation is a therapeutic goal of various autoimmune/autoinflammatory diseases including Behçet's disease (BD). The immunomodulatory effect of metabolites or metabolic analogs such as butyrate and 3-bromopyruvate has been observed in animal disease models.
OBJECTIVE
We attempted to evaluate the effect of butyrate and 3-bromopyruvate on the inflammatory cytokine production by peripheral blood mononuclear cells (PBMCs) isolated from patients with mucocutaneous involvement of BD.
METHODS
PBMCs isolated from 11 patients with BD and 10 healthy controls were stimulated with lipopolysaccharide in the presence of butyrate or 3-bromopyruvate. Butyrate receptor and cytokine messenger ribonucleic acid (mRNA) expression was analyzed by real-time reverse transcription polymerase chain reaction. Cytokine secretion was assessed by enzyme-linked immunosorbent assay. PBMCs survival was analyzed by flow cytometry.
RESULTS
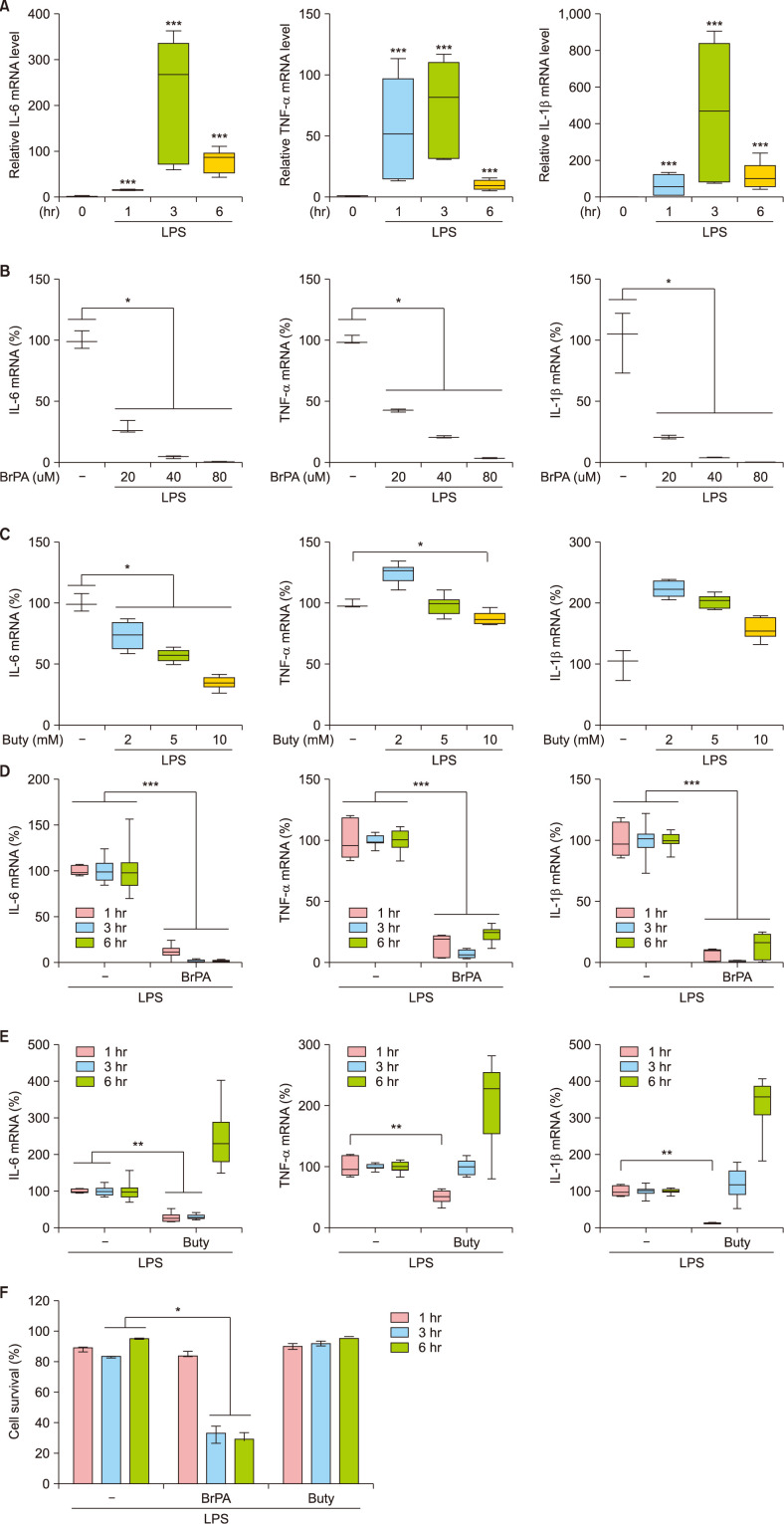
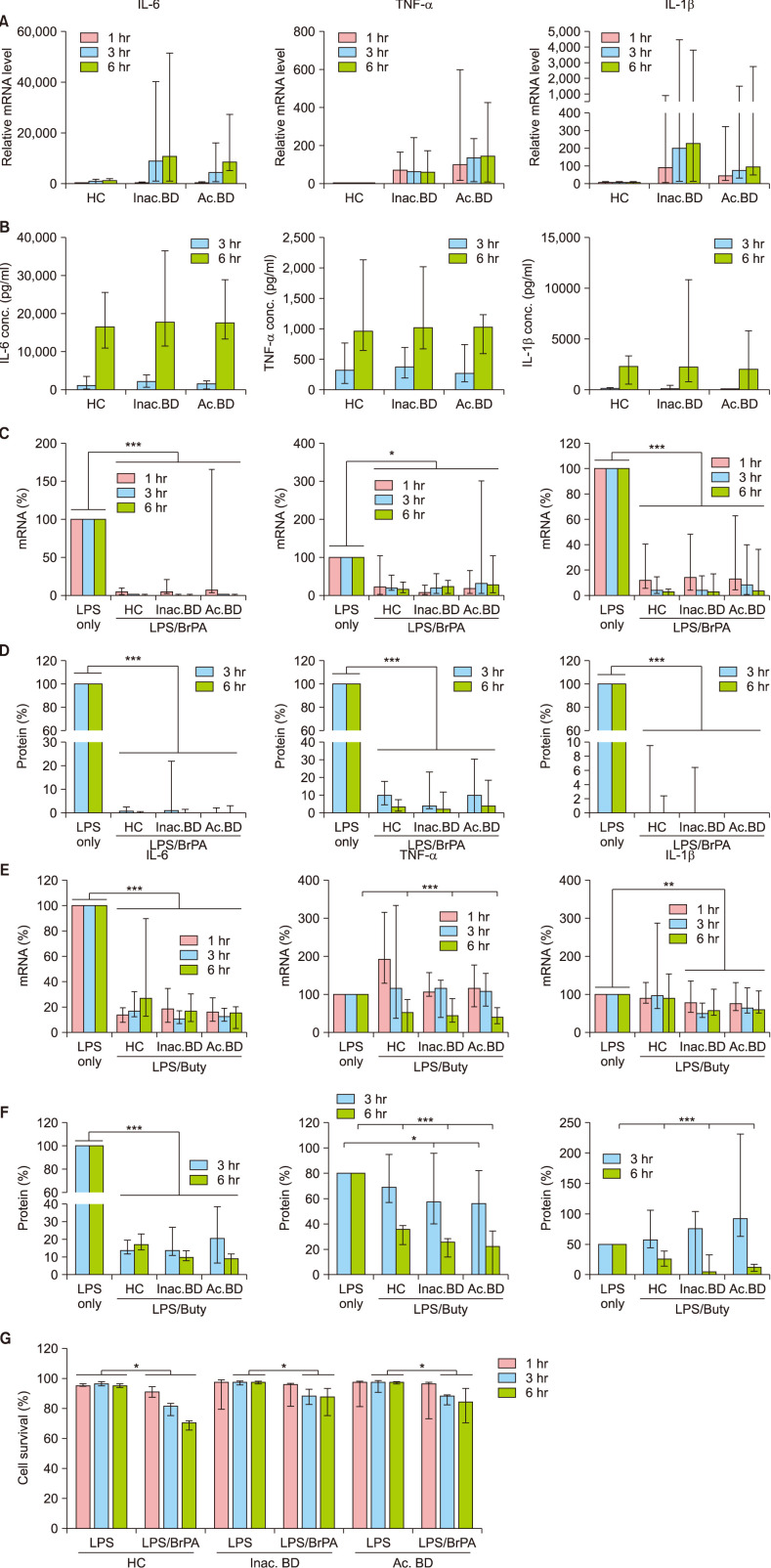
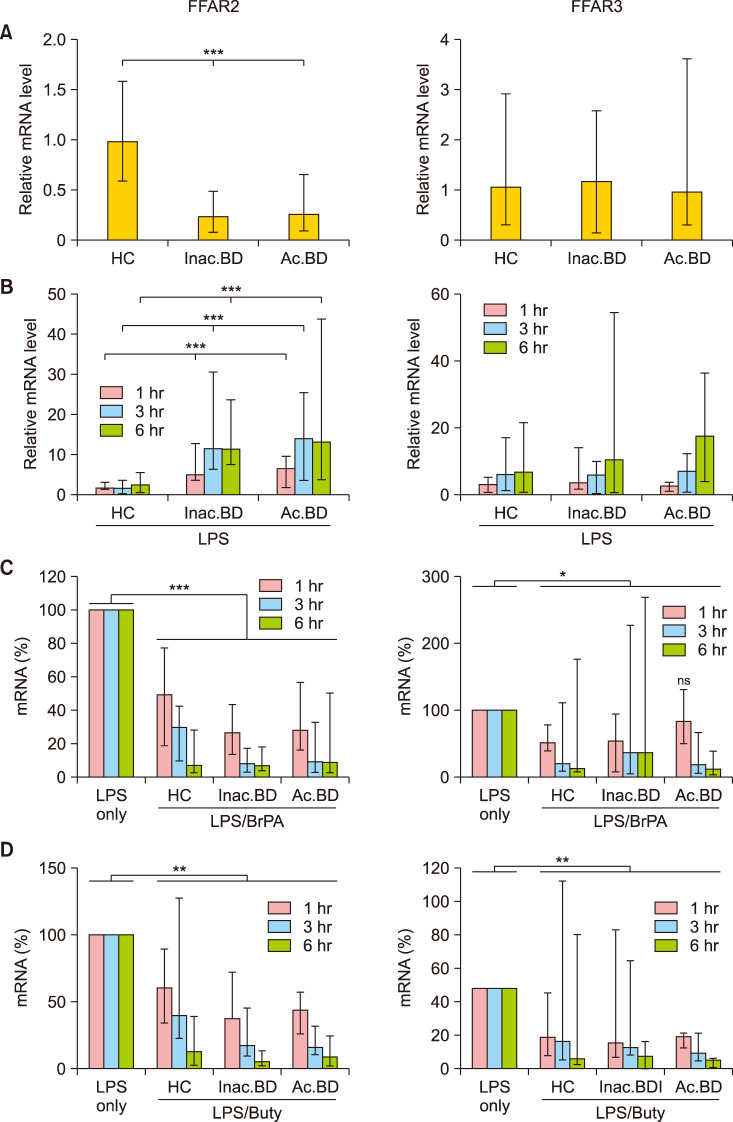
Bromopyruvate or butyrate treatment suppressed inflammatory cytokine production in PBMCs from all our subjects. Bromopyruvate also reduced PBMCs survival while butyrate did not. As the effect of butyrate was slightly greater in BD patients than in healthy controls, we analyzed butyrate receptor expression and found that lipopolysaccharide-induced free fatty acid receptor 2 mRNA level in PBMCs was higher in BD patients than in controls.
CONCLUSION
We propose bromopyruvate and butyrate as supplementary therapeutic candidates to control inflammation in patients with BD.
Keyword
MeSH Terms
Figure
Reference
-
1. Alpsoy E. Behçet's disease: a comprehensive review with a focus on epidemiology, etiology and clinical features, and management of mucocutaneous lesions. J Dermatol. 2016; 43:620–632. PMID: 27075942.
Article2. Loftus RM, Finlay DK. Immunometabolism: cellular metabolism turns immune regulator. J Biol Chem. 2016; 291:1–10. PMID: 26534957.
Article3. Seki SM, Stevenson M, Rosen AM, Arandjelovic S, Gemta L, Bullock TNJ, et al. Lineage-specific metabolic properties and vulnerabilities of T cells in the demyelinating central nervous system. J Immunol. 2017; 198:4607–4617. PMID: 28507026.
Article4. Okano T, Saegusa J, Nishimura K, Takahashi S, Sendo S, Ueda Y, et al. 3-bromopyruvate ameliorate autoimmune arthritis by modulating Th17/Treg cell differentiation and suppressing dendritic cell activation. Sci Rep. 2017; 7:42412. PMID: 28186160.
Article5. Schönfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res. 2016; 57:943–954. PMID: 27080715.
Article6. Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology. 2016; 5:e73. PMID: 27195116.
Article7. Consolandi C, Turroni S, Emmi G, Severgnini M, Fiori J, Peano C, et al. Behçet's syndrome patients exhibit specific microbiome signature. Autoimmun Rev. 2015; 14:269–276. PMID: 25435420.
Article8. Ko YH, Smith BL, Wang Y, Pomper MG, Rini DA, Torbenson MS, et al. Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun. 2004; 324:269–275. PMID: 15465013.
Article9. Lachmandas E, Boutens L, Ratter JM, Hijmans A, Hooiveld GJ, Joosten LA, et al. Microbial stimulation of different Toll-like receptor signalling pathways induces diverse metabolic programmes in human monocytes. Nat Microbiol. 2016; 2:16246. PMID: 27991883.
Article10. Nastasi C, Candela M, Bonefeld CM, Geisler C, Hansen M, Krejsgaard T, et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci Rep. 2015; 5:16148. PMID: 26541096.
Article11. Yuan X, Wang L, Bhat OM, Lohner H, Li PL. Differential effects of short chain fatty acids on endothelial Nlrp3 inflammasome activation and neointima formation: antioxidant action of butyrate. Redox Biol. 2018; 16:21–31. PMID: 29475132.
Article12. Säemann MD, Parolini O, Böhmig GA, Kelemen P, Krieger PM, Neumüller J, et al. Bacterial metabolite interference with maturation of human monocyte-derived dendritic cells. J Leukoc Biol. 2002; 71:238–246. PMID: 11818444.13. Chen G, Ran X, Li B, Li Y, He D, Huang B, et al. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine. 2018; 30:317–325. PMID: 29627390.
Article14. Geri G, Terrier B, Rosenzwajg M, Wechsler B, Touzot M, Seilhean D, et al. Critical role of IL-21 in modulating TH17 and regulatory T cells in Behçet disease. J Allergy Clin Immunol. 2011; 128:655–664. PMID: 21724243.15. Na SY, Park MJ, Park S, Lee ES. Up-regulation of Th17 and related cytokines in Behçet's disease corresponding to disease activity. Clin Exp Rheumatol. 2013; 31(3 Suppl 77):32–40. PMID: 24064012.16. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013; 504:451–455. PMID: 24226773.
Article17. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013; 504:446–450. PMID: 24226770.
Article18. Ko YH, Verhoeven HA, Lee MJ, Corbin DJ, Vogl TJ, Pedersen PL. A translational study “case report” on the small molecule “energy blocker” 3-bromopyruvate (3BP) as a potent anticancer agent: from bench side to bedside. J Bioenerg Biomembr. 2012; 44:163–170. PMID: 22328020.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Short-chain Fatty Acids Inhibit Staphylococcal Lipoprotein-induced Nitric Oxide Production in Murine Macrophages
- Sodium butyrate inhibits high glucose-induced inflammation by controlling the acetylation of NF-κB p65 in human monocytes
- 4-CMTB Ameliorates Ovalbumin-Induced Allergic Asthma through FFA2 Activation in Mice
- The Changes of Expression of Survivin by Butyrate in HCT116 Colon Cancer Cells
- The Role of Butyrate in Attenuating Pathobiont-Induced Hyperinflammation