Nutr Res Pract.
2023 Feb;17(1):164-173. 10.4162/nrp.2023.17.1.164.
Sodium butyrate inhibits high glucose-induced inflammation by controlling the acetylation of NF-κB p65 in human monocytes
- Affiliations
-
- 1Department of Food and Nutrition, Chonnam National University, Gwangju 61186, Korea
- KMID: 2539449
- DOI: http://doi.org/10.4162/nrp.2023.17.1.164
Abstract
- BACKGROUND/OBJECTIVES
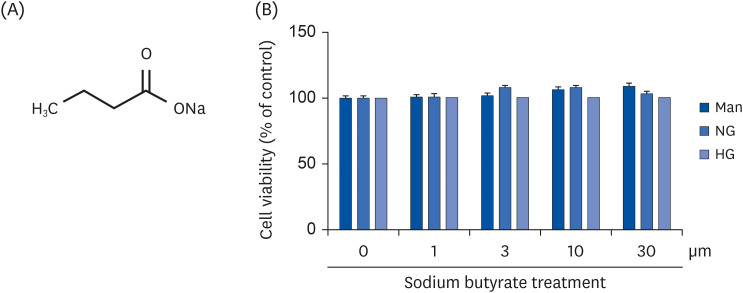
Hyperglycemia is a major cause of diabetes and diabetesrelated diseases. Sodium butyrate (NaB) is a short-chain fatty acid derivative that produces dietary fiber by anaerobic bacterial fermentation in the large intestine and occurs in foods, such as Parmesan cheese and butter. Butyrate has been shown to prevent obesity, improve insulin sensitivity, and ameliorate dyslipidemia in diet-induced obese mice. Therefore, this study examined the effects and mechanism of NaB on the secretion of inflammatory cytokines induced by high glucose (HG) in THP-1 cells.
MATERIALS/METHODS
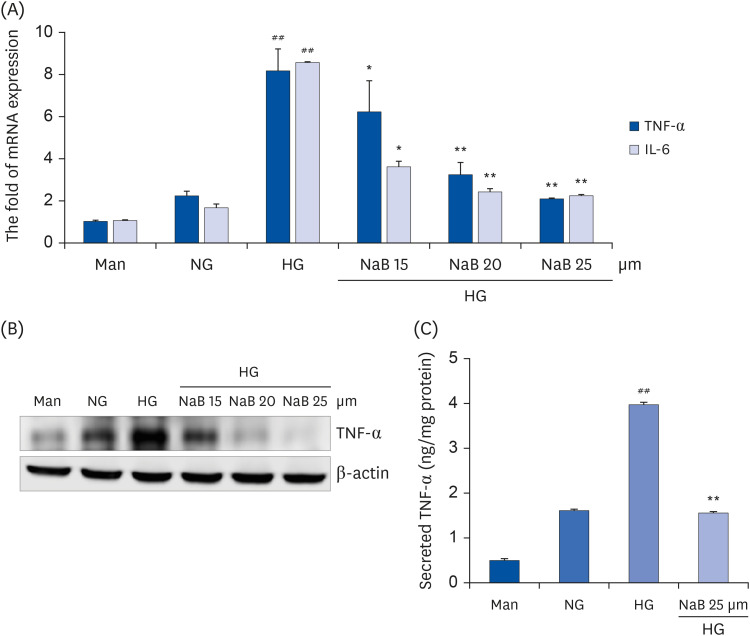
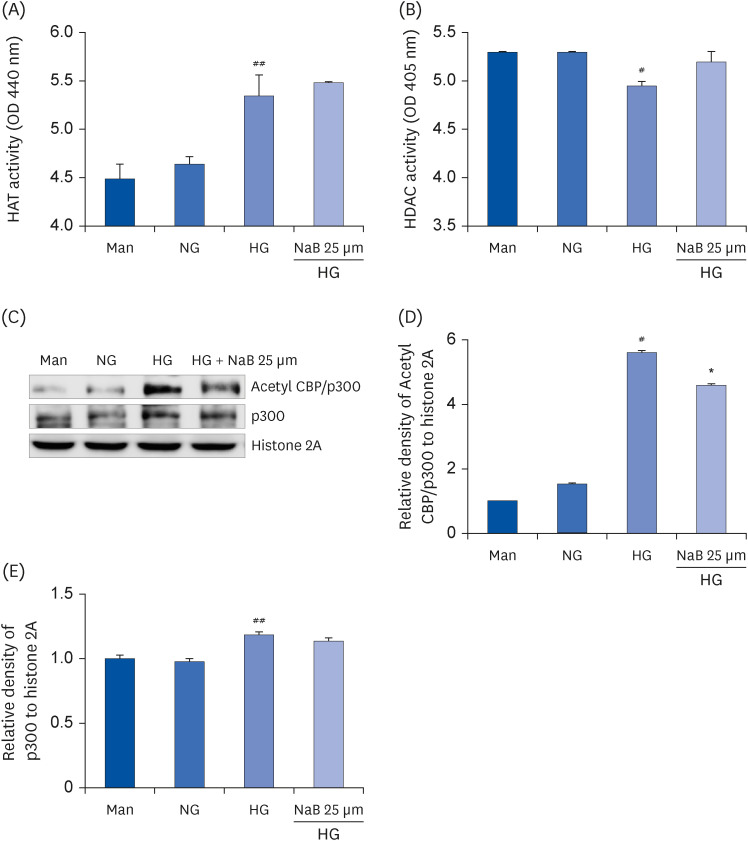
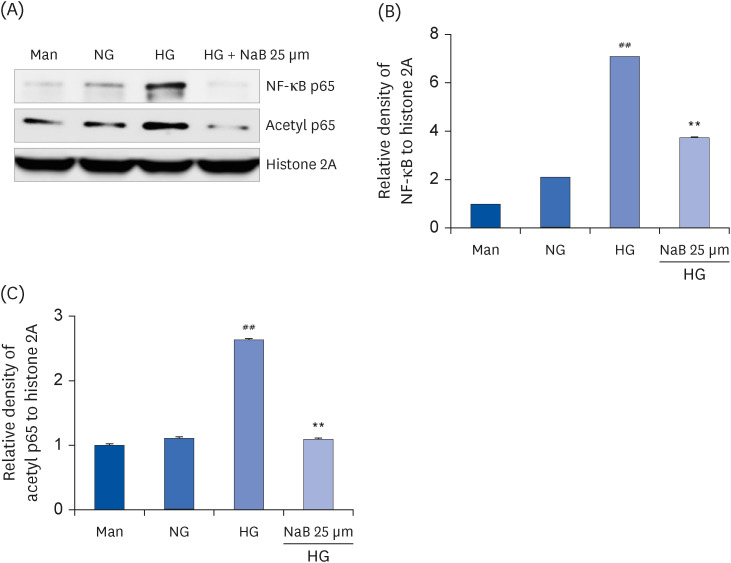
THP-1 cells were used as an in vitro model for HG-induced inflammation. The cells were cultured under normal glycemic or hyperglycemic conditions with or without NaB (0–25 μM). Western blotting and quantitative polymerase chain reaction were used to evaluate the protein and mRNA levels of nuclear factor-κB (NF-κB), interleukin-6, tumor necrosis factor-α, acetylated p65, acetyl CREB-binding protein/p300 (CBP/p300), and p300 using THP-1 cells. Histone acetyltransferase (HAT), histone deacetylase (HDAC), and pro-inflammatory cytokine secretion activity were analyzed using an enzyme-linked immunosorbent assay.
RESULTS
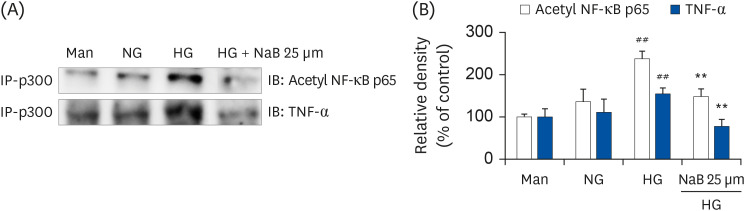
HG significantly upregulated histone acetylation, acetylation levels of p300, NF-κB activation, and inflammatory cytokine release in THP-1 cells. Conversely, the NaB treatment reduced cytokine release and NF-κB activation in HG-treated cells. It also significantly reduced p65 acetylation, CBP/p300 HAT activity, and CBP/p300 gene expression. In addition, NaB decreased the interaction of p300 in acetylated NF-κB and TNF-α.
CONCLUSIONS
These results suggest that NaB suppresses HG-induced inflammatory cytokine production through HAT/HDAC regulation in monocytes. NaB has the potential for preventing and treating diabetes and its related complications.
Figure
Reference
-
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JC, Mbanya JC, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022; 183:109119. PMID: 34879977.2. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014; 37(Suppl 1):S81–S90. PMID: 24357215.3. Kang JX, Weylandt KH. Modulation of inflammatory cytokines by omega-3 fatty acids. Subcell Biochem. 2008; 49:133–143. PMID: 18751910.4. Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014; 18:1–14. PMID: 24634591.5. Jialal I, Devaraj S, Venugopal SK. Oxidative stress, inflammation, and diabetic vasculopathies: the role of alpha tocopherol therapy. Free Radic Res. 2002; 36:1331–1336. PMID: 12607825.6. Kim DY, Kim SR, Jung UJ. Myricitrin ameliorates hyperglycemia, glucose intolerance, hepatic steatosis, and inflammation in high-fat diet/streptozotocin-induced diabetic mice. Int J Mol Sci. 2020; 21:1870. PMID: 32182914.7. Guo Y, Zhuang X, Huang Z, Zou J, Yang D, Hu X, Du Z, Wang L, Liao X. Klotho protects the heart from hyperglycemia-induced injury by inactivating ROS and NF-κB-mediated inflammation both in vitro and in vivo . Biochim Biophys Acta Mol Basis Dis. 2018; 1864:238–251. PMID: 28982613.8. Aryangat AV, Gerich JE. Type 2 diabetes: postprandial hyperglycemia and increased cardiovascular risk. Vasc Health Risk Manag. 2010; 6:145–155. PMID: 20448799.9. Nikiforov NG, Galstyan KO, Nedosugova LV, Elizova NV, Kolmychkova KI, Ivanova EA. Pro-inflammatory monocyte polarization in type 2 diabetes mellitus and coronary heart disease. Vessel Plus. 2017; 1:192–195.10. Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, Deftereos S, Tousoulis D. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. 2019; 14:50–59. PMID: 31131037.11. Yun JM, Surh J. Anti-inflammatory activity of onion juice prepared from sulfur-fertilized onions in high glucose induced human monocytes. Korean J Food Sci Technol. 2014; 46:773–777.12. Yun JM, Jialal I, Devaraj S. Epigenetic regulation of high glucose-induced proinflammatory cytokine production in monocytes by curcumin. J Nutr Biochem. 2011; 22:450–458. PMID: 20655188.13. Miao F, Gonzalo IG, Lanting L, Natarajan R. In vivo chromatin remodeling events leading to inflammatory gene transcription under diabetic conditions. J Biol Chem. 2004; 279:18091–18097. PMID: 14976218.14. Wegner M, Neddermann D, Piorunska-Stolzmann M, Jagodzinski PP. Role of epigenetic mechanisms in the development of chronic complications of diabetes. Diabetes Res Clin Pract. 2014; 105:164–175. PMID: 24814876.15. Lee W, Lee SY, Son YJ, Yun JM. Gallic acid decreases inflammatory cytokine secretion through histone acetyltransferase/histone deacetylase regulation in high glucose-induced human monocytes. J Med Food. 2015; 18:793–801. PMID: 25807193.16. Spange S, Wagner T, Heinzel T, Krämer OH. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int J Biochem Cell Biol. 2009; 41:185–198. PMID: 18804549.17. Kim HJ, Kim SH, Yun JM. Fisetin inhibits hyperglycemia-induced proinflammatory cytokine production by epigenetic mechanisms. Evid Based Complement Alternat Med. 2012; 2012:639469. PMID: 23320034.18. Shakespear MR, Halili MA, Irvine KM, Fairlie DP, Sweet MJ. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 2011; 32:335–343. PMID: 21570914.19. Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010; 28:1057–1068. PMID: 20944598.20. Khullar M, Cheema BS, Raut SK. Emerging evidence of epigenetic modifications in vascular complication of diabetes. Front Endocrinol (Lausanne). 2017; 8:237. PMID: 29085333.21. Pham TX, Lee J. Dietary regulation of histone acetylases and deacetylases for the prevention of metabolic diseases. Nutrients. 2012; 4:1868–1886. PMID: 23363995.22. Saldanha SN, Kala R, Tollefsbol TO. Molecular mechanisms for inhibition of colon cancer cells by combined epigenetic-modulating epigallocatechin gallate and sodium butyrate. Exp Cell Res. 2014; 324:40–53. PMID: 24518414.23. Khan S, Jena GB. Protective role of sodium butyrate, a HDAC inhibitor on beta-cell proliferation, function and glucose homeostasis through modulation of p38/ERK MAPK and apoptotic pathways: study in juvenile diabetic rat. Chem Biol Interact. 2014; 213:1–12. PMID: 24530320.24. Ruderman NB, Williamson JR, Brownlee M. Glucose and diabetic vascular disease. FASEB J. 1992; 6:2905–2914. PMID: 1644256.25. Ashburner BP, Westerheide SD, Baldwin AS Jr. The p65 (RelA) subunit of NF-κB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol Cell Biol. 2001; 21:7065–7077. PMID: 11564889.26. Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Klöting I, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-κB. Diabetes. 2001; 50:2792–2808. PMID: 11723063.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eriodictyol Inhibits the Production and Gene Expression of MUC5AC Mucin via the IκBα-NF-κB p65 Signaling Pathway in Airway Epithelial Cells
- Sweroside plays a role in mitigating high glucose-induced damage in human renal tubular epithelial HK-2 cells by regulating the SIRT1/NF-κB signaling pathway
- Protective effects and mechanism of coenzyme Q10 and vitamin C on doxorubicin-induced gastric mucosal injury and effects of intestinal flora
- Hydroxychavicol Inhibits In Vitro Osteoclastogenesis via the Suppression of NF-κB Signaling Pathway
- Kaempferol Regulates the Expression of Airway MUC5AC Mucin Gene via IκBα-NF-κB p65 and p38-p44/42-Sp1 Signaling Pathways