Allergy Asthma Immunol Res.
2018 Sep;10(5):516-532. 10.4168/aair.2018.10.5.516.
Lactobacillus plantarum-derived Extracellular Vesicles Protect Atopic Dermatitis Induced by Staphylococcus aureus-derived Extracellular Vesicles
- Affiliations
-
- 1Department of Internal Medicine, Ewha Womans University College of Medicine, Seoul, Korea. mineyang81@ewha.ac.kr
- 2Research Institute, AEON Medix, Inc., Pohang, Korea.
- 3Department of Life Science, Division of Molecular and Life Sciences, Pohang University of Science and Technology, Pohang, Korea.
- 4Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
- 5Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea.
- 6Institute of MD Healthcare, Inc., Seoul, Korea.
- 7CJ R&D Center, CJ CheilJedang Corporation, Suwon, Korea.
- 8Pediatric Allergy and Respiratory Center, Department of Pediatrics, Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea. bypyun@schmc.ac.kr
- 9Department of Computer Science and Engineering, Hanyang University, Seoul, Korea.
- 10Department of Internal Medicine, National Medical Center, Seoul, Korea.
- KMID: 2418049
- DOI: http://doi.org/10.4168/aair.2018.10.5.516
Abstract
- PURPOSE
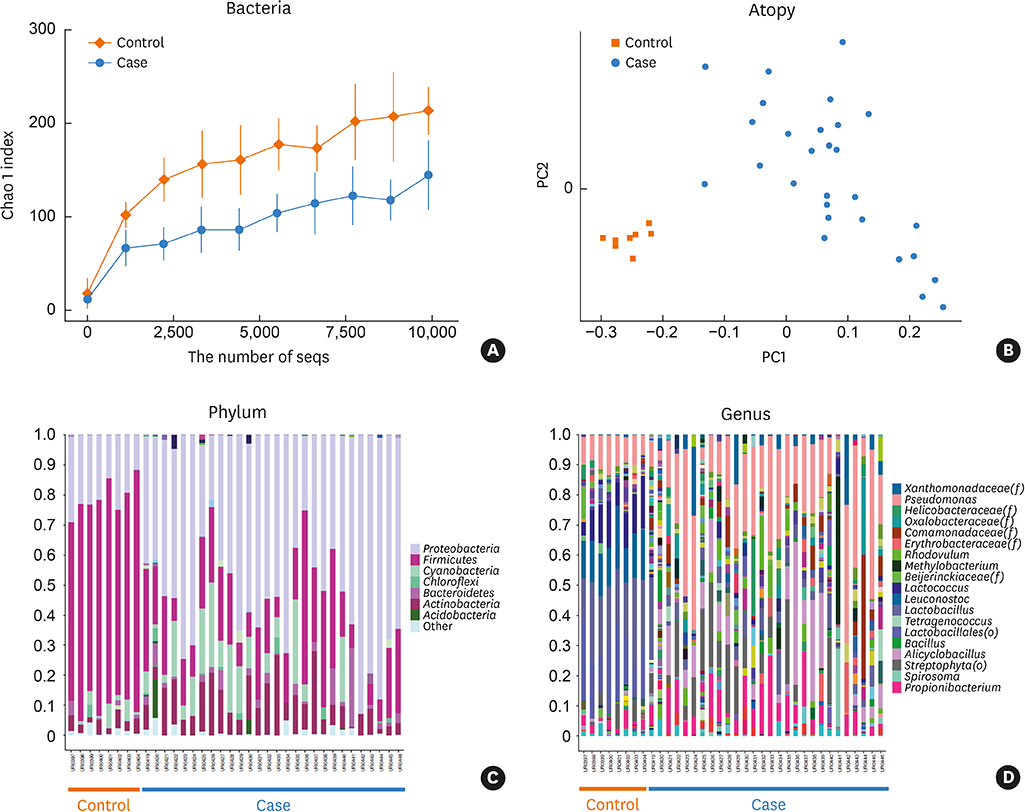
The microbial environment is an important factor that contributes to the pathogenesis of atopic dermatitis (AD). Recently, it was revealed that not only bacteria itself but also extracellular vesicles (EVs) secreted from bacteria affect the allergic inflammation process. However, almost all research carried out so far was related to local microorganisms, not the systemic microbial distribution. We aimed to compare the bacterial EV composition between AD patients and healthy subjects and to experimentally find out the beneficial effect of some bacterial EV composition
METHODS
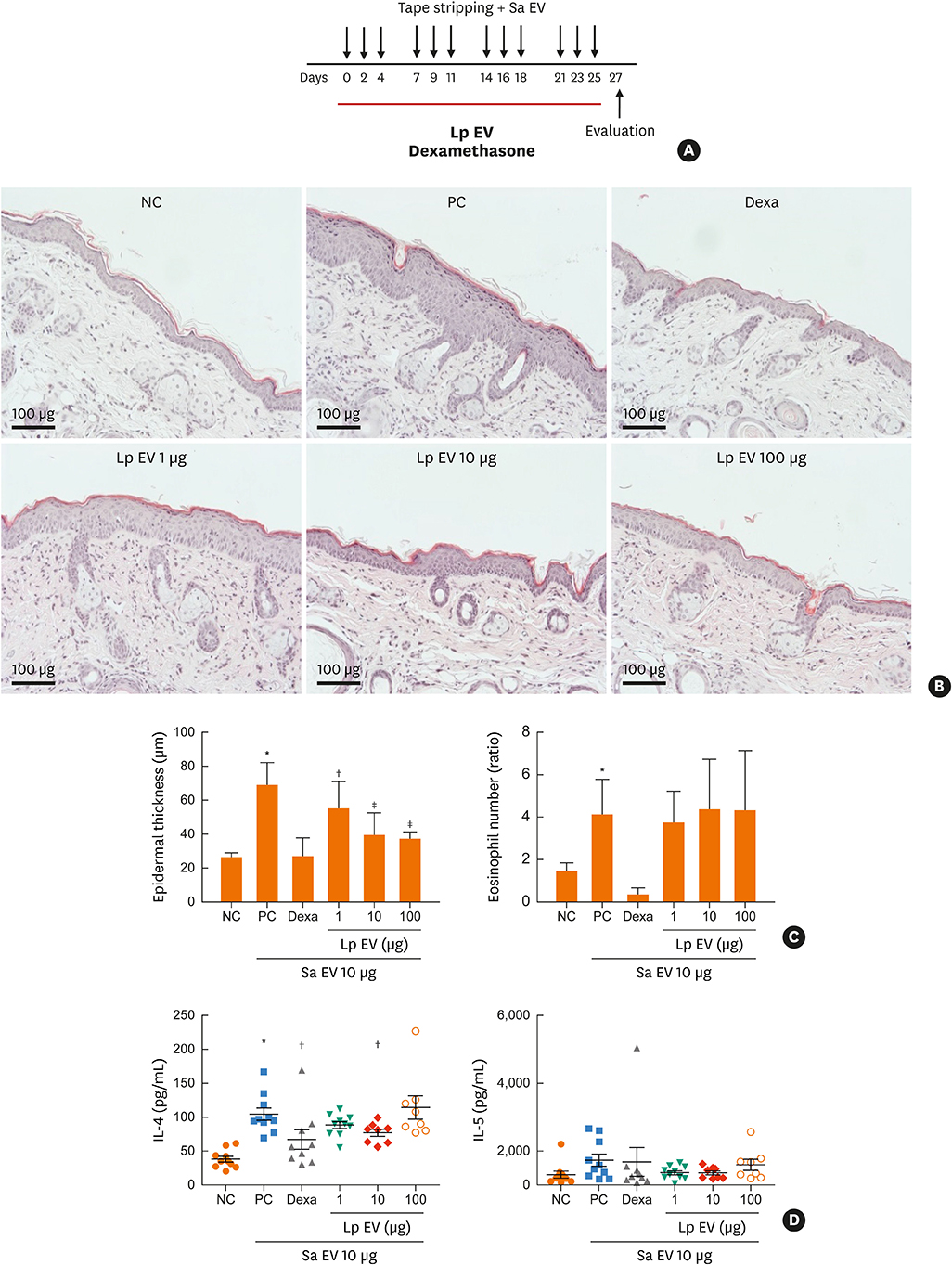
Twenty-seven AD patients and 6 healthy control subjects were enrolled. After urine and serum were obtained, EVs were prepared from samples. Metagenomic analysis of 16s ribosomal DNA extracted from the EVs was performed, and bacteria showing the greatest difference between controls and patients were identified. In vitro and in vivo therapeutic effects of significant bacterial EV were evaluated with keratinocytes and with Staphylococcus aureus-induced mouse AD models, respectively.
RESULTS
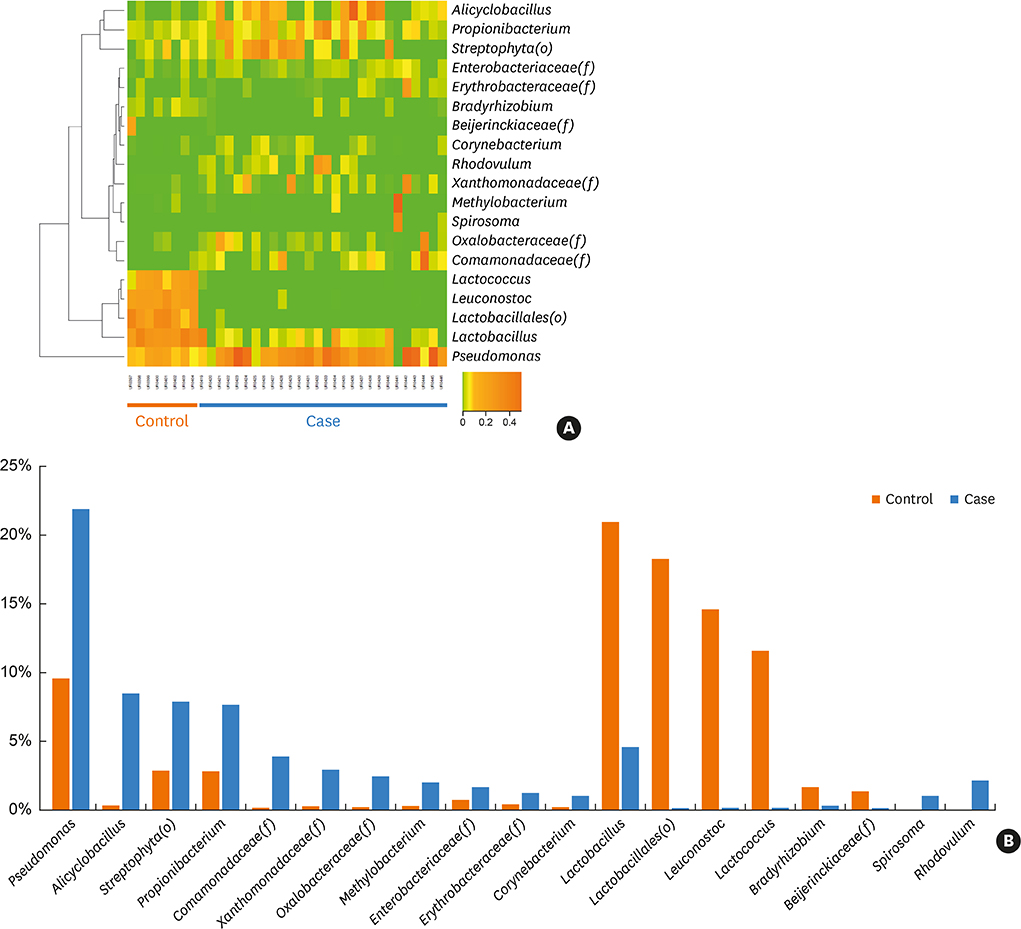
The proportions of Lactococcus, Leuconostoc and Lactobacillus EVs were significantly higher and those of Alicyclobacillus and Propionibacterium were lower in the control group than in the AD patient group. Therefore, lactic acid bacteria were considered to be important ones that contribute to the difference between the patient and control groups. In vitro, interleukin (IL)-6 from keratinocytes and macrophages decreased and cell viability was restored with Lactobacillus plantarum-derived EV treatment prior to S. aureus EV treatment. In S. aureus-induced mouse AD models, L. plantarum-derived EV administration reduced epidermal thickening and the IL-4 level.
CONCLUSIONS
We suggested the protective role of lactic acid bacteria in AD based on metagenomic analysis. Experimental findings further suggest that L. plantarum-derived EV could help prevent skin inflammation.
Keyword
MeSH Terms
-
Alicyclobacillus
Animals
Bacteria
Cell Survival
Dermatitis, Atopic*
DNA, Ribosomal
Extracellular Vesicles*
Healthy Volunteers
Humans
In Vitro Techniques
Inflammation
Interleukin-4
Interleukins
Keratinocytes
Lactic Acid
Lactobacillus*
Lactococcus
Leuconostoc
Macrophages
Metagenomics
Mice
Microbiota
Probiotics
Propionibacterium
Skin
Staphylococcus*
Therapeutic Uses
DNA, Ribosomal
Interleukin-4
Interleukins
Lactic Acid
Therapeutic Uses
Figure
Reference
-
1. Sabin BR, Peters N, Peters AT. Chapter 20: atopic dermatitis. Allergy Asthma Proc. 2012; 33:Suppl 1. 67–69.
Article2. Bieber T. Atopic dermatitis. N Engl J Med. 2008; 358:1483–1494.
Article3. Pyun BY. Natural history and risk factors of atopic dermatitis in children. Allergy Asthma Immunol Res. 2015; 7:101–105.
Article4. Ahn K. The prevalence of atopic dermatitis in Korean children. Allergy Asthma Immunol Res. 2016; 8:1–2.
Article5. Hong SJ, Ahn KM, Lee SY, Kim KE. The prevalences of asthma and allergic diseases in Korean children. Korean J Pediatr. 2008; 51:343–350.
Article6. Lee JH, Han KD, Kim KM, Park YG, Lee JY, Park YM. Prevalence of atopic dermatitis in Korean children based on data from the 2008–2011 Korean National Health and Nutrition Examination Survey. Allergy Asthma Immunol Res. 2016; 8:79–83.
Article7. Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004; 113:651–657.
Article8. Boguniewicz M, Leung DY. 10. Atopic dermatitis. J Allergy Clin Immunol. 2006; 117:S475–S480.
Article9. Jang SC, Kim SR, Yoon YJ, Park KS, Kim JH, Lee J, et al. In vivo kinetic biodistribution of nano-sized outer membrane vesicles derived from bacteria. Small. 2015; 11:456–461.10. Kim JH, Lee J, Park J, Gho YS. Gram-negative and gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol. 2015; 40:97–104.
Article11. Shin TS, Kim JH, Kim YS, Jeon SG, Zhu Z, Gho YS, et al. Extracellular vesicles are key intercellular mediators in the development of immune dysfunction to allergens in the airways. Allergy. 2010; 65:1256–1265.
Article12. Admyre C, Telemo E, Almqvist N, Lötvall J, Lahesmaa R, Scheynius A, et al. Exosomes - nanovesicles with possible roles in allergic inflammation. Allergy. 2008; 63:404–408.
Article13. Mathieu A, Vogel TM, Simonet P. The future of skin metagenomics. Res Microbiol. 2014; 165:69–76.
Article14. Eisen JA. Environmental shotgun sequencing: its potential and challenges for studying the hidden world of microbes. PLoS Biol. 2007; 5:e82.
Article15. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980; 60:Suppl 92. 44–47.16. Severity scoring of atopic dermatitis: the SCORAD index. Consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1993; 186:23–31.17. Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, et al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009; 9:5425–5436.18. Vitaliti G, Pavone P, Guglielmo F, Spataro G, Falsaperla R. The immunomodulatory effect of probiotics beyond atopy: an update. J Asthma. 2014; 51:320–332.
Article19. von der Weid T, Bulliard C, Schiffrin EJ. Induction by a lactic acid bacterium of a population of CD4(+) T cells with low proliferative capacity that produce transforming growth factor beta and interleukin-10. Clin Diagn Lab Immunol. 2001; 8:695–701.20. Smits HH, Engering A, van der Kleij D, de Jong EC, Schipper K, van Capel TM, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005; 115:1260–1267.21. Rosenfeldt V, Benfeldt E, Nielsen SD, Michaelsen KF, Jeppesen DL, Valerius NH, et al. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol. 2003; 111:389–395.22. West CE, Hammarström ML, Hernell O. Probiotics in primary prevention of allergic disease--follow-up at 8–9 years of age. Allergy. 2013; 68:1015–1020.23. Yang HJ, Min TK, Lee HW, Pyun BY. Efficacy of probiotic therapy on atopic dermatitis in children: a randomized, double-blind, placebo-controlled trial. Allergy Asthma Immunol Res. 2014; 6:208–215.
Article24. Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev. 2007; CD006475.
Article25. Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, Murrell DF, Tang ML. Probiotics for treating eczema. Cochrane Database Syst Rev. 2008; CD006135.
Article26. Niccoli AA, Artesi AL, Candio F, Ceccarelli S, Cozzali R, Ferraro L, et al. Preliminary results on clinical effects of probiotic Lactobacillus salivarius LS01 in children affected by atopic dermatitis. J Clin Gastroenterol. 2014; 48:Suppl 1. S34–S36.27. Won TJ, Kim B, Lee Y, Bang JS, Oh ES, Yoo JS, et al. Therapeutic potential of Lactobacillus plantarum CJLP133 for house-dust mite-induced dermatitis in NC/Nga mice. Cell Immunol. 2012; 277:49–57.28. Won TJ, Kim B, Lim YT, Song DS, Park SY, Park ES, et al. Oral administration of Lactobacillus strains from kimchi inhibits atopic dermatitis in NC / Nga mice. J Appl Microbiol. 2011; 110:1195–1202.29. Han Y, Kim B, Ban J, Lee J, Kim BJ, Choi BS, et al. A randomized trial of Lactobacillus plantarum CJLP133 for the treatment of atopic dermatitis. Pediatr Allergy Immunol. 2012; 23:667–673.30. Yamamoto M, Haruna T, Yasui K, Takahashi H, Iduhara M, Takaki S, et al. A novel atopic dermatitis model induced by topical application with Dermatophagoides farinae extract in NC/Nga mice. Allergol Int. 2007; 56:139–148.31. Kim H, Kim HR, Kim NR, Jeong BJ, Lee JS, Jang S, et al. Oral administration of Lactobacillus plantarum lysates attenuates the development of atopic dermatitis lesions in mouse models. J Microbiol. 2015; 53:47–52.32. Jeong YI, Hong SH, Cho SH, Lee WJ, Lee SE. Toxoplasma gondii infection suppresses house dust mite extract-induced atopic dermatitis in NC/Nga mice. Allergy Asthma Immunol Res. 2015; 7:557–564.33. Pyun BY. Extracellular vesicle: an unknown environmental factor for causing airway disease. Allergy Asthma Immunol Res. 2016; 8:179–180.
Article34. Choi Y, Park H, Park HS, Kim YK. Extracellular vesicles, a key mediator to link environmental microbiota to airway immunity. Allergy Asthma Immunol Res. 2017; 9:101–106.
Article35. Kim MH, Rho M, Choi JP, Choi HI, Park HK, Song WJ, et al. A metagenomic analysis provides a culture-independent pathogen detection for atopic dermatitis. Allergy Asthma Immunol Res. 2017; 9:453–461.
Article36. Findley K, Grice EA. The skin microbiome: a focus on pathogens and their association with skin disease. PLoS Pathog. 2014; 10:e1004436.
Article37. Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009; 324:1190–1192.
Article38. Butler-Wu SM, Burns EM, Pottinger PS, Magaret AS, Rakeman JL, Matsen FA 3rd, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011; 49:2490–2495.39. Ege MJ, Mayer M, Schwaiger K, Mattes J, Pershagen G, van Hage M, et al. Environmental bacteria and childhood asthma. Allergy. 2012; 67:1565–1571.
Article40. Fyhrquist N, Ruokolainen L, Suomalainen A, Lehtimäki S, Veckman V, Vendelin J, et al. Acinetobacter species in the skin microbiota protect against allergic sensitization and inflammation. J Allergy Clin Immunol. 2014; 134:1301–1309.e11.41. Brand S, Teich R, Dicke T, Harb H, Yildirim AO, Tost J, et al. Epigenetic regulation in murine offspring as a novel mechanism for transmaternal asthma protection induced by microbes. J Allergy Clin Immunol. 2011; 128:618–625. 625.e1–625.e7.
Article42. Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence? Br J Dermatol. 2008; 158:442–455.
Article43. Forsythe P, Inman MD, Bienenstock J. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. Am J Respir Crit Care Med. 2007; 175:561–569.44. Sjögren YM, Jenmalm MC, Böttcher MF, Björkstén B, Sverremark-Ekström E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin Exp Allergy. 2009; 39:518–526.
Article45. Kang H, Oh YJ, Ahn KS, Eom HJ, Han N, Kim YB, et al. Leuconostoc citreum HJ-P4 (KACC 91035) regulates immunoglobulin E in an ovalbumin-induced allergy model and induces interleukin-12 through nuclear factor-kappa B and p38/c-Jun N-terminal kinases signaling in macrophages. Microbiol Immunol. 2009; 53:331–339.46. Hong SW, Kim MR, Lee EY, Kim JH, Kim YS, Jeon SG, et al. Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy. 2011; 66:351–359.47. Hong SW, Choi EB, Min TK, Kim JH, Kim MH, Jeon SG, et al. An important role of α-hemolysin in extracellular vesicles on the development of atopic dermatitis induced by Staphylococcus aureus . PLoS One. 2014; 9:e100499.48. Kim YS, Choi JP, Kim MH, Park HK, Yang S, Kim YS, et al. IgG sensitization to extracellular vesicles in indoor dust is closely associated with the prevalence of non-eosinophilic asthma, COPD, and lung cancer. Allergy Asthma Immunol Res. 2016; 8:198–205.
Article49. Grice EA, Kong HH, Renaud G, Young AC. A diversity profile of the human skin microbiota. Genome Res. 2008; 18:1043–1050.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Extracellular Vesicles Derived from Lactobacillus plantarum Increase BDNF Expression in Cultured Hippocampal Neurons and Produce Antidepressant-like Effects in Mice
- Trends in Developing Extracellular Vesicle-Based Therapeutics
- Staphylococcus aureus Membrane Vesicles and Its Potential Role in Bacterial Pathogenesis
- Extracellular Vesicles and Immune System in Ageing and Immune Diseases
- Extracellular Vesicles of Neutrophils