J Vet Sci.
2018 May;19(3):331-338. 10.4142/jvs.2018.19.3.331.
Association between endotoxin levels in dust from indoor swine housing environments and the immune responses of pigs
- Affiliations
-
- 1Department of Occupational Health, College of Bio-Medical Sciences, Daegu Catholic University, Gyeongsan 38430, Korea. yheo@cu.ac.kr
- 2Technology Services Division, National Institute of Animal Science, Wanju 55365, Korea.
- 3Dodram Pig Farmer's Cooperative, Veterinary Service Center, Daejeon 35352, Korea.
- 4Department of Preventive Medicine, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea. kimha@catholic.ac.kr
- KMID: 2412123
- DOI: http://doi.org/10.4142/jvs.2018.19.3.331
Abstract
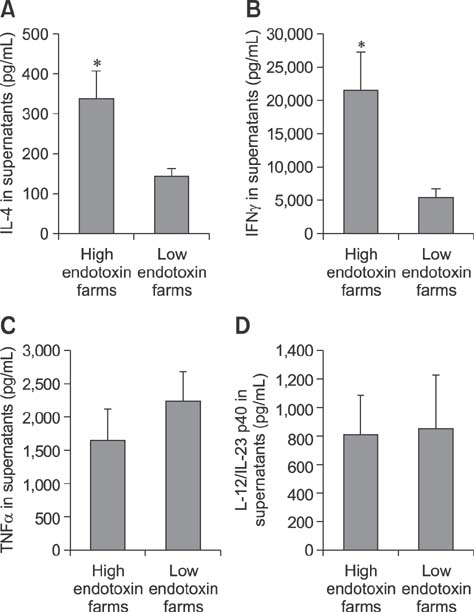
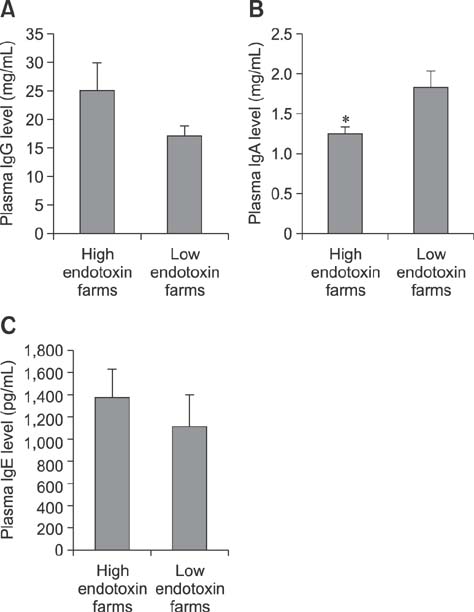
- Indoor animal husbandry environments are inevitably contaminated with endotoxins. Endotoxin exposure is associated with various inflammatory illnesses in animals. This cross-sectional study evaluated the relationship between the degree of endotoxin exposure and the cellular and humoral immune profiles of fattening pigs. Blood samples were taken from the jugular vein of 47 pigs from ten pig farms in Korea. Whole blood cell counts and plasma immunoglobulin (Ig) classes were determined. Peripheral-blood mononuclear cells were stimulated in vitro with concanavalin A for 48 h, and cytokines released into culture supernatants were measured. The barns in which the pigs lived were assessed for endotoxin levels in the total and respirable dust by using the limulus amebocyte lysate kinetic QCL method. Low and high endotoxin exposures were defined as ≤ 30 and > 30 EU/m³, respectively. Compared to pigs with low endotoxin exposure (n = 19), highly exposed pigs (n = 28) had higher circulating neutrophil and lymphocyte (particularly B cells) counts, IgG and IgE levels, interferon-gamma (IFNγ) and interleukin (IL)-4 productions, and lower IgA levels and tumor necrosis factor-alpha (TNFα) production. The IL-4, IFNγ, and TNFα levels significantly correlated with endotoxin level and/or pig age. Constant exposure of pigs to high levels of airborne endotoxins can lead to aberrant immune profiles.
Keyword
MeSH Terms
-
Agriculture
Animal Husbandry
Animals
Blood Cell Count
Concanavalin A
Cross-Sectional Studies
Cytokines
Dust*
Endotoxins
Horseshoe Crabs
Housing*
Immunity, Cellular
Immunoglobulin A
Immunoglobulin E
Immunoglobulin G
Immunoglobulins
In Vitro Techniques
Interferon-gamma
Interleukin-4
Interleukins
Jugular Veins
Korea
Lymphocytes
Methods
Neutrophils
Plasma
Swine*
Tumor Necrosis Factor-alpha
Concanavalin A
Cytokines
Dust
Endotoxins
Immunoglobulin A
Immunoglobulin E
Immunoglobulin G
Immunoglobulins
Interferon-gamma
Interleukin-4
Interleukins
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Alexis NE, Lay JC, Almond M, Peden DB. Inhalation of low-dose endotoxin favors local TH2 response and primes airway phagocytes in vivo. J Allergy Clin Immunol. 2004; 114:1325–1331.
Article2. American Conference of Governmental Industrial Hygienists (ACGIH). TLVs and BEIs: 2010. Cincinnati: ACGIH Worldwide;2010. p. 74–77.3. Bakutis B, Monstviliene G, Januskeviciene G. Analyses of airborne contamination with bacteria, endotoxins and dust in livestock barns and poultry houses. Acta Vet Brno. 2004; 73:283–289.
Article4. Barboza R, Câmara NO, Gomes E, Sá-Nunes A, Florsheim E, Mirotti L, Labrada A, Alcântara-Neves NM, Russo M. Endotoxin exposure during sensitization to Blomia tropicalis allergens shifts TH2 immunity towards a TH17-mediated airway neutrophilic inflammation: role of TLR4 and TLR2. PLoS One. 2013; 8:e67115.5. Castegren M, Skorup P, Lipcsey M, Larsson A, Sjölin J. Endotoxin tolerance variation over 24 h during porcine endotoxemia: association with changes in circulation and organ dysfunction. PLoS One. 2013; 8:e53221.
Article6. Charavaryamath C, Keet T, Aulakh GK, Townsend HG, Singh B. Lung responses to secondary endotoxin challenge in rats exposed to pig barn air. J Occup Med Toxicol. 2008; 3:24.
Article7. Costa A, Borgonovo F, Leroy T, Berckmans D, Guarino M. Dust concentration variation in relation to animal activity in a pig barn. Biosyst Eng. 2009; 104:118–124.
Article8. Daiwen C, Keying Z, Chunyan W. Influences of lipopolysaccharide-induced immune challenge on performance and whole-body protein turnover in weanling pigs. Livest Sci. 2008; 113:291–295.
Article9. Darwich L, Segalés J, Domingo M, Mateu E. Changes in CD4+, CD8+, CD4+ CD8+, and immunoglobulin M-positive peripheral blood mononuclear cells of postweaning multisystemic wasting syndrome-affected pigs and age-matched uninfected wasted and healthy pigs correlate with lesions and porcine circovirus type 2 load in lymphoid tissues. Clin Diagn Lab Immunol. 2002; 9:236–236.10. Egawa G, Weninger W. Pathogenesis of atopic dermatitis: a short review. Cogent Biol. 2015; 1:1103459.
Article11. Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002; 196:1645–1651.
Article12. Halloy DJ, Kirschvink NA, Mainil J, Gustin PG. Synergistic action of E. coli endotoxin and Pasteurella multocida type A for the induction of bronchopneumonia in pigs. Vet J. 2005; 169:417–426.
Article13. Health Council of the Netherlands. Endotoxins: Health-based Recommended Occupational Exposure Limit. Hague: Health Council of the Netherlands;2010.14. Heo Y, Lee WT, Lawrence DA. Differential effects of lead and cAMP on development and activities of Th1- and Th2-lymphocytes. Toxicol Sci. 1998; 43:172–185.
Article15. Iqbal S, Zebeli Q, Mansmann DA, Dunn SM, Ametaj BN. Repeated oronasal exposure to lipopolysaccharide induced mucosal IgA responses in periparturient dairy cows. PLoS One. 2014; 9:e103504.
Article16. Kim HA, Kim JY, Shin KM, Jo JH, Roque K, Jo GH, Heo Y. Relationship between endotoxin level of in swine farm dust and cellular immunity of husbandry workers. J Korean Soc Occup Environ Hyg. 2013; 23:393–401.17. Knetter SM, Tuggle CK, Wannemuehler MJ, Ramer-Tait AE. Organic barn dust extract exposure impairs porcine macrophage function in vitro: implications for respiratory health. Vet Immunol Immunopathol. 2014; 157:20–30.
Article18. Kullik K, Brosig B, Kersten S, Valenta H, Diesing AK, Panther P, Reinhardt N, Kluess J, Rothkötter HJ, Breves G, Dänicke S. Interactions between the Fusarium toxin deoxynivalenol and lipopolysaccharides on the in vivo protein synthesis of acute phase proteins, cytokines and metabolic activity of peripheral blood mononuclear cells in pigs. Food Chem Toxicol. 2013; 57:11–20.
Article19. Kwon KS, Lee IB, Hwang HS, Hong SW, Seo IH, Ha TH, Ha JS, Park HA. Measurement and analysis of aerosols in swine confined house for welfare improvement of workers. In : Proceedings of the KSAM & KSBEC 2013 Spring Conference; 3 May 2013; Yesan, Korea. p. 107–108.20. Latza U, Oldenburg M, Baur X. Endotoxin exposure and respiratory symptoms in the cotton textile industry. Arch Environ Health. 2004; 59:519–525.
Article21. Liu AH. Something old, something new: indoor endotoxin, allergens and asthma. Paediatr Respir Rev. 2004,; 5:Suppl 1. S65–S71.
Article22. May S, Romberger DJ, Poole JA. Respiratory health effects of large animal farming environments. J Toxicol Environ Health B Crit Rev. 2012; 15:524–541.
Article23. McAleer JP, Vella AT. Understanding how lipopolysaccharide impacts CD4 T-cell immunity. Crit Rev Immunol. 2008; 28:281–299.
Article24. Merck Veterinary Manual. Hematologic Reference Ranges-Appendixes. Kenilworth: Merck Sharp & Dohme;2018.25. Milton DK, Wypij D, Kriebel D, Walters MD, Hammond SK, Evans JS. Endotoxin exposure-response in a fiberglass manufacturing facility. Am J Ind Med. 1996; 29:3–13.
Article26. Ministry of Agriculture, Food and Rural Affairs (MAFRA). Guideline on Environmental Friendly Animal Husbandry. Sejong: MAFRA;2008.27. Myers MJ, Farrell DE, Palmer DC, Post LO. Inflammatory mediator production in swine following endotoxin challenge with or without co-administration of dexamethasone. Int Immunopharmacol. 2003; 3:571–579.
Article28. Park JH, Spiegelman DL, Burge HA, Gold DR, Chew GL, Milton DK. Longitudinal study of dust and airborne endotoxin in the home. Environ Health Perspect. 2000; 108:1023–1028.
Article29. Poole JA, Alexis NE, Parks C, MacInnes AK, Gentry-Nielsen MJ, Fey PD, Larsson L, Allen-Gipson D, Von Essen SG, Romberger DJ. Repetitive organic dust exposure in vitro impairs macrophage differentiation and function. J Allergy Clin Immunol. 2008; 122:375–382.30. Preller L, Heederik D, Kromhout H, Boleij JS, Tielen MJ. Determinants of dust and endotoxin exposure of pig farmers: development of a control strategy using empirical modelling. Ann Occup Hyg. 1995; 39:545–557.
Article31. Qiao S, Feng L, Bao D, Guo J, Wan B, Xiao Z, Yang S, Zhang G. Porcine reproductive and respiratory syndrome virus and bacterial endotoxin act in synergy to amplify the inflammatory response of infected macrophages. Vet Microbiol. 2011; 149:213–220.
Article32. Radon K, Danuser B, Iversen M, Monso E, Weber C, Hartung J, Donham K, Palmgren U, Nowak D. Air contaminants in different European farming environments. Ann Agric Environ Med. 2002; 9:41–48.33. Roque K, Lim GD, Jo JH, Shin KM, Song ES, Gautam R, Kim CY, Lee K, Shin S, Yoo HS, Heo Y, Kim HA. Epizootiological characteristics of viable bacteria and fungi in indoor air from porcine, chicken, or bovine husbandry confinement buildings. J Vet Sci. 2016; 17:531–538.
Article34. Roque K, Lim GD, Song ES, Gautam R, Lee JH, Kim YG, Cho AR, Shin SJ, Kim CY, Kim HA, Heo Y. Association of bovine cellular immunity with endotoxin level in dust from Korean beef cattle housing environments. Quant Bio-Sci. 2016; 35:61–66.
Article35. Roque K, Shin KM, Jo JH, Kim HA, Heo Y. Relationship between chicken cellular immunity and endotoxin levels in dust from chicken housing environments. J Vet Sci. 2015; 16:173–177.
Article36. Schierl R, Heise A, Egger U, Schneider F, Eichelser R, Neser S, Nowak D. Endotoxin concentration in modern animal houses in southern Bavaria. Ann Agric Environ Med. 2007; 14:129–136.37. Wang AX, Xu Landén N. New insights into T cells and their signature cytokines in atopic dermatitis. IUBMB Life. 2015; 67:601–610.
Article38. Wunschel J, Poole JA. Occupational agriculture organic dust exposure and its relationship to asthma and airway inflammation in adults. J Asthma. 2016; 53:471–477.
Article39. Wysocka M, Robertson S, Riemann H, Caamano J, Hunter C, Mackiewicz A, Montaner LJ, Trinchieri G, Karp CL. IL-12 suppression during experimental endotoxin tolerance: dendritic cell loss and macrophage hyporesponsiveness. J Immunol. 2001; 166:7504–7513.
Article40. Zhang Y, Zhou X, Zhou B. DC-derived TSLP promotes Th2 polarization in LPS-primed allergic airway inflammation. Eur J Immunol. 2012; 42:1735–1743.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship between chicken cellular immunity and endotoxin levels in dust from chicken housing environments
- Immunopathogenesis of Allergic Asthma: More Than the Th2 Hypothesis
- Epizootiological characteristics of viable bacteria and fungi in indoor air from porcine, chicken, or bovine husbandry confinement buildings
- Endotoxin and House Dust Mite Allergen Levels on Synthetic and Buckwheat Pillows
- Effect of a Resident and Indoor Environmental Characteristics on the House Dust Mites Allergen