Korean J Physiol Pharmacol.
2018 Mar;22(2):163-172. 10.4196/kjpp.2018.22.2.163.
Effects of salmon DNA fraction in vitro and in a monosodium iodoacetate-induced osteoarthritis rat model
- Affiliations
-
- 1Department of Orthopedic Surgery, Gangneung Asan Hospital, Ulsan University College of Medicine, Gangneung 25440, Korea.
- 2Medical Research Institute, Gangneung Asan Hospital, Gangneung 25440, Korea.
- 3Department of Pathology, Gangneung Asan Hospital, Ulsan University College of Medicine, Gangneung 25440, Korea.
- 4Natural Products Research Institute, Korea Institute of Science and Technology, Gangneung 25440, Korea.
- 5Department of Orthopedic Surgery, Hanil General Hospital, Seoul 01450, Korea.
- 6Department of Surgery, Gangneung Asan Hospital, Ulsan University College of Medicine, Gangneung 25440, Korea. d990081@gmail.com
- KMID: 2410106
- DOI: http://doi.org/10.4196/kjpp.2018.22.2.163
Abstract
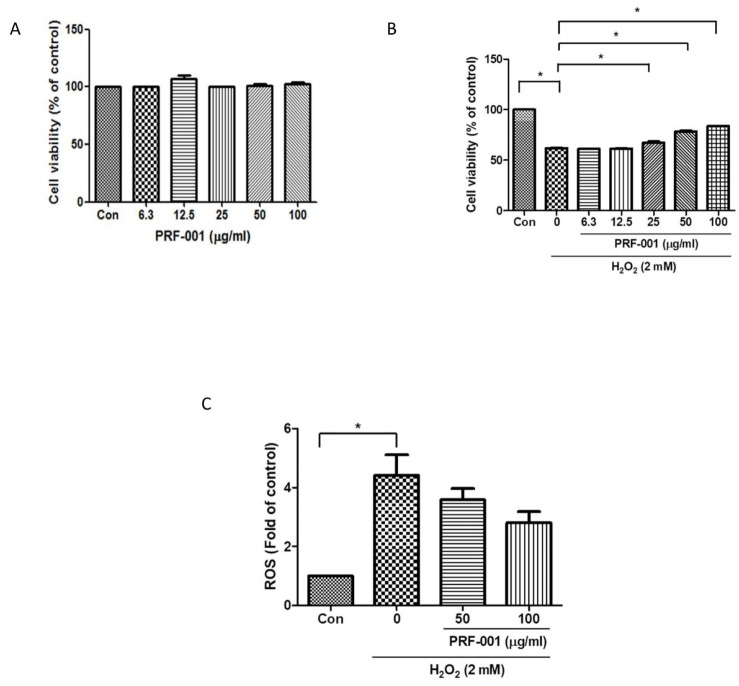
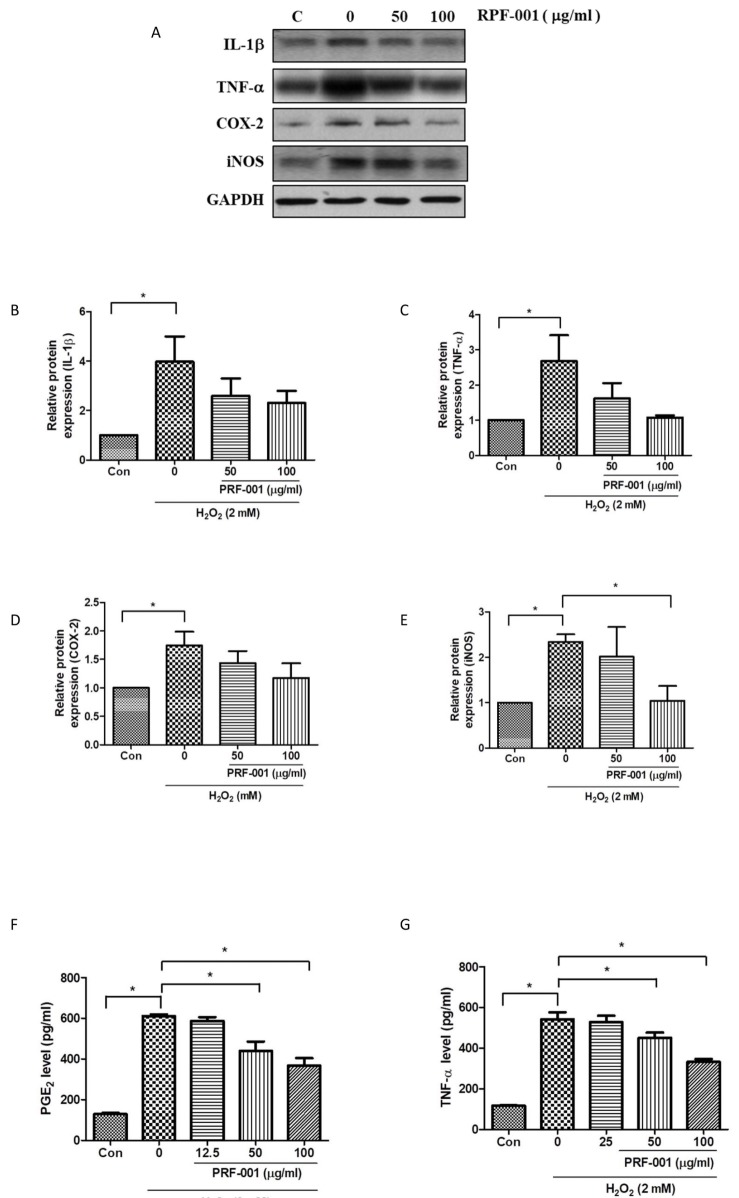
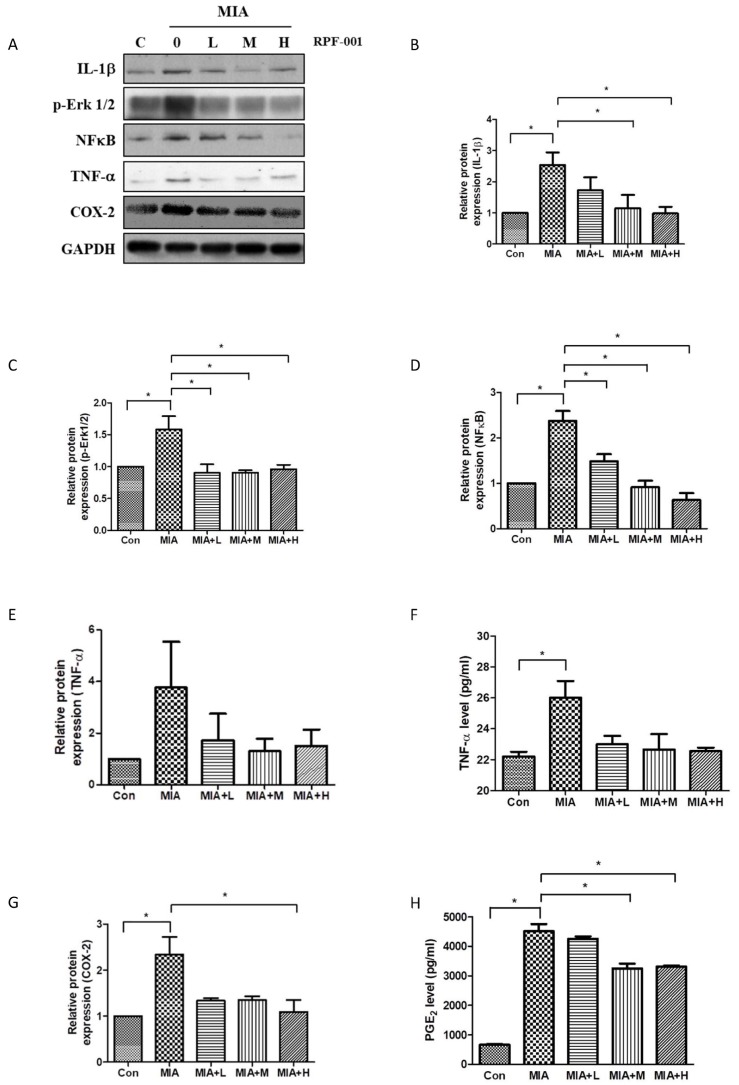
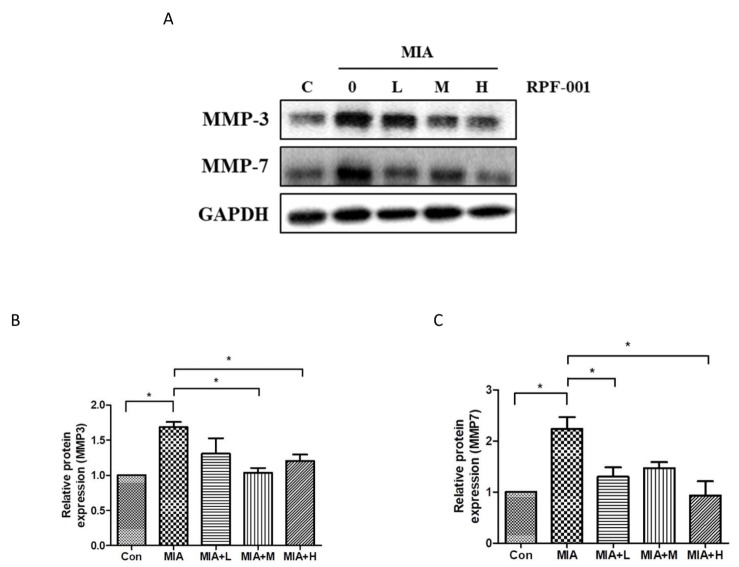
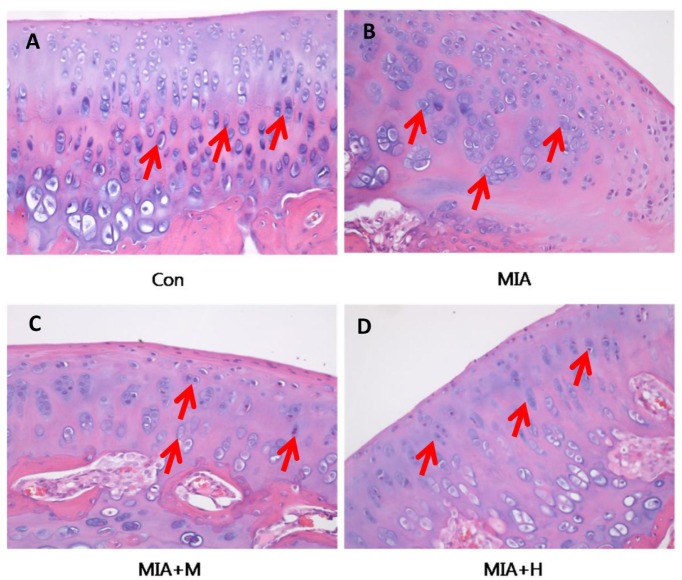
- PRF001 is a fragmented DNA polymer extracted from the testes of salmon. The purpose of this study was to assess the anti-inflammatory effect of PRF001 in vitro as well as the protective effect of PRF001 intake against arthritis in a rat model. In vitro, cell survival and inflammatory markers after H₂O₂ treatment to induce cell damage were investigated in CHON-001 cells treated with different concentrations of PRF001. In vivo, osteoarthritis was induced by intra-articular injection of monosodium iodoacetate (MIA) into the knee joints of rats. After consumption of PRF001 (10, 50, or 100 mg/kg) for 4 weeks, inflammatory mediators and cytokines in articular cartilage were investigated. In vitro, the levels of inflammatory markers, IL-1β, TNF-α, COX-2, iNOS, and PGE2, were significantly suppressed by PRF001 treatment. In vivo, the inflammatory mediators and cytokines, IL-1β, p-Erk1/2, NF-κB, TNF-α, COX-2, and PGE2, as well as MMP3 and MMP7, which have catabolic activity in chondrocytes, were decreased in the MIA-induced osteoarthritic rats following intake of PRF001. Histological analysis revealed that PRF001 had a protective effect on the articular cartilage. Altogether, these results demonstrated that the anti-inflammatory property of PRF001 contributes to its protective effects in osteoarthritis through deregulating IL-1β, TNF-α, and subsequent signals, such as p-Erk1/2, NF-κB, COX-2, PGE2, and MMPs.
MeSH Terms
Figure
Reference
-
1. Johnston SA. Osteoarthritis. Joint anatomy, physiology, and pathobiology. Vet Clin North Am Small Anim Pract. 1997; 27:699–723. PMID: 9243777.2. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, Howell D, Kaplan D, Koopman W, Longley S III, Mankin H, McShane DJ, Medsger T Jr, Meenan R, Mikkelsen W, Moskowitz R, Murphy W, Rothschild B, Segal M, Sokoloff L, Wolfe F. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986; 29:1039–1049. PMID: 3741515.3. Wearing SC, Hennig EM, Byrne NM, Steele JR, Hills AP. Musculoskeletal disorders associated with obesity: a biomechanical perspective. Obes Rev. 2006; 7:239–250. PMID: 16866972.
Article4. Campo GM, Avenoso A, Campo S, D'Ascola A, Traina P, Samà D, Calatroni A. Glycosaminoglycans modulate inflammation and apoptosis in LPS-treated chondrocytes. J Cell Biochem. 2009; 106:83–92. PMID: 19009563.
Article5. Herrington C, Hall PA. Molecular and cellular themes in inflammation and immunology. J Pathol. 2008; 214:123–125. PMID: 18161749.
Article6. Wu W, Xu X, Dai Y, Xia L. Therapeutic effect of the saponin fraction from Clematis chinensis Osbeck roots on osteoarthritis induced by monosodium iodoacetate through protecting articular cartilage. Phytother Res. 2010; 24:538–546. PMID: 19655297.
Article7. Henderson B, Pettipher ER. Arthritogenic actions of recombinant IL-1 and tumour necrosis factor alpha in the rabbit: evidence for synergistic interactions between cytokines in vivo. Clin Exp Immunol. 1989; 75:306–310. PMID: 2784740.8. Mabey T, Honsawek S. Cytokines as biochemical markers for knee osteoarthritis. World J Orthop. 2015; 6:95–105. PMID: 25621214.
Article9. Massicotte F, Lajeunesse D, Benderdour M, Pelletier JP, Hilal G, Duval N, Martel-Pelletier J. Can altered production of interleukin-1beta, interleukin-6, transforming growth factor-beta and prostaglandin E(2) by isolated human subchondral osteoblasts identify two subgroups of osteoarthritic patients. Osteoarthritis Cartilage. 2002; 10:491–500. PMID: 12056853.10. Page Thomas DP, King B, Stephens T, Dingle JT. In vivo studies of cartilage regeneration after damage induced by catabolin/interleukin-1. Ann Rheum Dis. 1991; 50:75–80. PMID: 1998394.
Article11. Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002; 39:237–246. PMID: 12082286.12. Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000; 2:459–465. PMID: 11123098.
Article13. Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004; (427 Suppl):S27–S36. PMID: 15480070.
Article14. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011; 7:33–42. PMID: 21119608.
Article15. Park B, Prasad S, Yadav V, Sung B, Aggarwal BB. Boswellic acid suppresses growth and metastasis of human pancreatic tumors in an orthotopic nude mouse model through modulation of multiple targets. PLoS One. 2011; 6:e26943. PMID: 22066019.
Article16. Roy S, Khanna S, Krishnaraju AV, Subbaraju GV, Yasmin T, Bagchi D, Sen CK. Regulation of vascular responses to inflammation: inducible matrix metalloproteinase-3 expression in human microvascular endothelial cells is sensitive to antiinflammatory Boswellia. Antioxid Redox Signal. 2006; 8:653–660. PMID: 16677108.17. Sengupta K, Kolla JN, Krishnaraju AV, Yalamanchili N, Rao CV, Golakoti T, Raychaudhuri S, Raychaudhuri SP. Cellular and molecular mechanisms of anti-inflammatory effect of Aflapin: a novel Boswellia serrata extract. Mol Cell Biochem. 2011; 354:189–197. PMID: 21479939.
Article18. Garner BC, Stoker AM, Kuroki K, Evans R, Cook CR, Cook JL. Using animal models in osteoarthritis biomarker research. J Knee Surg. 2011; 24:251–264. PMID: 22303754.
Article19. Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA, Griffin MR, Moskowitz RW, Schnitzer TJ. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum. 1995; 38:1541–1546. PMID: 7488273.20. Gottlieb D, Kuritzky L. Using the medical food flavocoxid in managing osteoarthritis. J Pain Palliat Care Pharmacother. 2011; 25:49–54. PMID: 21426218.
Article21. Akyol S, Yükselten Y, Çakmak Ö, Ugurcu V, Altuntas A, Gürler M, Akyol Ö, Demircan K. Hydrogen peroxide-induced oxidative damage in human chondrocytes: the prophylactic effects of hypericum perforatum linn extract on deoxyribonucleic acid damage, apoptosis and matrix remodeling by a disintegrin-like and metalloproteinase with thrombospondin motifs proteinases. Arch Rheumatol. 2014; 29:203–214.
Article22. Tonello G, Daglio M, Zaccarelli N, Sottofattori E, Mazzei M, Balbi A. Characterization and quantitation of the active polynucleotide fraction (PDRN) from human placenta, a tissue repair stimulating agent. J Pharm Biomed Anal. 1996; 14:1555–1560. PMID: 8877863.
Article23. Lee DW, Hong HJ, Roh H, Lee WJ. The effect of polydeoxyribonucleotide on ischemic rat skin flap survival. Ann Plast Surg. 2015; 75:84–90. PMID: 25954843.
Article24. Squadrito F, Bitto A, Altavilla D, Arcoraci V, De Caridi G, De Feo ME, Corrao S, Pallio G, Sterrantino C, Minutoli L, Saitta A, Vaccaro M, Cucinotta D. The effect of PDRN, an adenosine receptor A2A agonist, on the healing of chronic diabetic foot ulcers: results of a clinical trial. J Clin Endocrinol Metab. 2014; 99:E746–E753. PMID: 24483158.25. Héraud F, Héraud A, Harmand MF. Apoptosis in normal and osteoarthritic human articular cartilage. Ann Rheum Dis. 2000; 59:959–965. PMID: 11087699.26. López-Armada MJ, Caramés B, Lires-Deán M, Cillero-Pastor B, Ruiz-Romero C, Galdo F, Blanco FJ. Cytokines, tumor necrosis factor-alpha and interleukin-1beta, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthritis Cartilage. 2006; 14:660–669. PMID: 16492401.27. Ye Z, Chen Y, Zhang R, Dai H, Zeng C, Zeng H, Feng H, Du G, Fang H, Cai D. c-Jun N-terminal kinase - c-Jun pathway transactivates Bim to promote osteoarthritis. Can J Physiol Pharmacol. 2014; 92:132–139. PMID: 24502636.
Article28. Altavilla D, Bitto A, Polito F, Marini H, Minutoli L, Di Stefano V, Irrera N, Cattarini G, Squadrito F. Polydeoxyribonucleotide (PDRN): a safe approach to induce therapeutic angiogenesis in peripheral artery occlusive disease and in diabetic foot ulcers. Cardiovasc Hematol Agents Med Chem. 2009; 7:313–321. PMID: 19860658.
Article29. Bitto A, Polito F, Altavilla D, Minutoli L, Migliorato A, Squadrito F. Polydeoxyribonucleotide (PDRN) restores blood flow in an experimental model of peripheral artery occlusive disease. J Vasc Surg. 2008; 48:1292–1300. PMID: 18971038.
Article30. Marcu KB, Otero M, Olivotto E, Borzi RM, Goldring MB. NFkappaB signaling: multiple angles to target OA. Curr Drug Targets. 2010; 11:599–613. PMID: 20199390.31. El Mansouri FE, Chabane N, Zayed N, Kapoor M, Benderdour M, Martel-Pelletier J, Pelletier JP, Duval N, Fahmi H. Contribution of H3K4 methylation by SET-1A to interleukin-1-induced cyclooxygenase 2 and inducible nitric oxide synthase expression in human osteoarthritis chondrocytes. Arthritis Rheum. 2011; 63:168–179. PMID: 20862685.
Article32. Hardy MM, Seibert K, Manning PT, Currie MG, Woerner BM, Edwards D, Koki A, Tripp CS. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis Rheum. 2002; 46:1789–1803. PMID: 12124863.
Article33. Jones SW, Brockbank SM, Clements KM, Le Good N, Campbell D, Read SJ, Needham MR, Newham P. Mitogen-activated protein kinase-activated protein kinase 2 (MK2) modulates key biological pathways associated with OA disease pathology. Osteoarthritis Cartilage. 2009; 17:124–131. PMID: 18562219.
Article34. Martel-Pelletier J, Pelletier JP, Fahmi H. Cyclooxygenase-2 and prostaglandins in articular tissues. Semin Arthritis Rheum. 2003; 33:155–167. PMID: 14671726.
Article35. Sakao K, Takahashi KA, Arai Y, Saito M, Honjo K, Hiraoka N, Asada H, Shin-Ya M, Imanishi J, Mazda O, Kubo T. Osteoblasts derived from osteophytes produce interleukin-6, interleukin-8, and matrix metalloproteinase-13 in osteoarthritis. J Bone Miner Metab. 2009; 27:412–423. PMID: 19333684.
Article36. Husa M, Liu-Bryan R, Terkeltaub R. Shifting HIFs in osteoarthritis. Nat Med. 2010; 16:641–664. PMID: 20526316.
Article37. Tao Y, Qiu X, Xu C, Sun B, Shi C. Expression and correlation of matrix metalloproteinase-7 and interleukin-15 in human osteoarthritis. Int J Clin Exp Pathol. 2015; 8:9112–9118. PMID: 26464654.38. Pitcher T, Sousa-Valente J, Malcangio M. The monoiodoacetate model of osteoarthritis pain in the mouse. J Vis Exp. 2016; (111):e53746.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corrigendum to: Effects of salmon DNA fraction in vitro and in a monosodium iodoacetate-induced osteoarthritis rat model
- Anti-osteoarthritis effect of Boswellia serrata gum resin extract in monosodium iodoacetate-induced osteoarthritic Sprague-Dawley rats
- Prevention or treatment of enzyme treated royal jelly on monosodium-iodoacetate-induced osteoarthritis
- Micro-CT Arthrographic Analysis of Monosodium Iodoacetate-Induced Osteoarthritis in Rat Knees
- Anti-inflammatory effect of egg white-chalcanthite and purple bamboo salts mixture on arthritis induced by monosodium iodoacetate in Sprague-Dawley rats