Ann Surg Treat Res.
2017 Oct;93(4):217-224. 10.4174/astr.2017.93.4.217.
The inflammatory response of neutrophils in an in vitro model that approximates the postcardiac arrest state
- Affiliations
-
- 1Department of Emergency Medicine, Korea University College of Medicine, Seoul, Korea.
- 2The institute for Trauma Research, Korea University, Seoul, Korea. kuedchoi@korea.ac.kr
- 3Department of Laboratory Medicine, Korea University College of Medicine, Seoul, Korea.
- KMID: 2392372
- DOI: http://doi.org/10.4174/astr.2017.93.4.217
Abstract
- PURPOSE
Postcardiac arrest syndrome (PCAS) shares many features with sepsis including plasma cytokine elevation with dysregulation of cytokine production, and the presence of endotoxin in plasma. PCAS is closely related to ischemia-reperfusion injury. During ischemia-reperfusion injury, neutrophil, which is the first line of innate immunity, plays a major role. In this study, we investigated the inflammatory response of human neutrophils in an in vitro model which we simulated with hypoxia-normoxia and hypoxia-hyperoxia environments.
METHODS
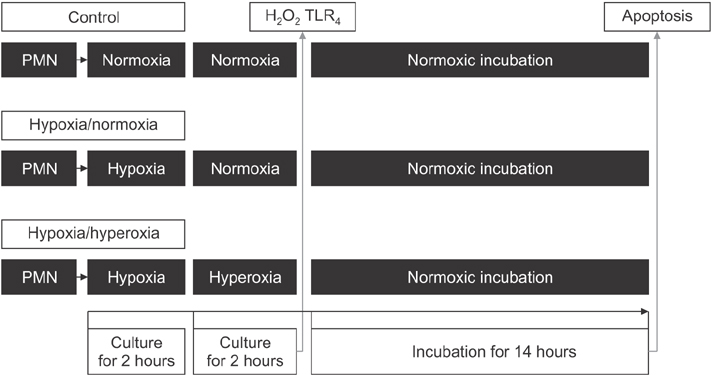
After separation of neutrophils from the whole blood, they were divided into 3 experimental groups: normoxia-normoxia, hypoxia-normoxia, and hypoxia-hyperoxia groups. The production of Hâ‚‚Oâ‚‚, the expression of Toll-like receptor 4 (TLRâ‚„) receptor, and the extent of apoptosis of the neutrophils were checked.
RESULTS
The in vitro hypoxia-normoxia and -hyperoxia models, which simulated the PCAS, showed initiation of the neutrophils' inflammatory reaction by hypoxia insult. Lipopolysaccharide amplifies such inflammation; therefore, prevention of secondary infection may be critical in postresuscitation patients. Temporary hyperoxia following hypoxic insult showed no difference in inflammatory reaction compared with hypoxia-normoxia. Rather, temporary hyperoxia may suppress or minimize inflammation by attenuation of TLR4 receptor.
CONCLUSION
It is well known that continuous hyperoxygenation after successful cardiac arrest harms patients, but temporary hyperoxygenation with 100% Oâ‚‚ in a clinical situation may be helpful.
Keyword
MeSH Terms
Figure
Reference
-
1. Negovsky VA. The second step in resuscitation--the treatment of the ‘post-resuscitation disease’. Resuscitation. 1972; 1:1–7.2. Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002; 106:562–568.3. Ar'Rajab A, Dawidson I, Fabia R. Reperfusion injury. New Horiz. 1996; 4:224–234.4. Strieter RM, Kasahara K, Allen RM, Standiford TJ, Rolfe MW, Becker FS, et al. Cytokine-induced neutrophil-derived interleukin-8. Am J Pathol. 1992; 141:397–407.5. Adrie C, Laurent I, Monchi M, Cariou A, Dhainaou JF, Spaulding C. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome. Curr Opin Crit Care. 2004; 10:208–212.6. Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002; 23:301–304.7. Prabhakar NR, Kumar GK. Oxidative stress in the systemic and cellular responses to intermittent hypoxia. Biol Chem. 2004; 385:217–221.8. Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002; 2:965–975.9. Francois S, El Benna J, Dang PM, Pedruzzi E, Gougerot-Pocidalo MA, Elbim C. Inhibition of neutrophil apoptosis by TLR agonists in whole blood: involvement of the phosphoinositide 3-kinase/Akt and NF-kappaB signaling pathways, leading to increased levels of Mcl-1, A1, and phosphorylated Bad. J Immunol. 2005; 174:3633–3642.10. Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006; 311:1–16.11. Eker T, Genc V, Sevim Y, Cumaogullari O, Ozcelik M, Kocaay AF, et al. The effects of ventilation with high density oxygen on the strength of gastrointestinal anastomosis. Ann Surg Treat Res. 2015; 89:17–22.12. Wood JG, Johnson JS, Mattioli LF, Gonzalez NC. Systemic hypoxia increases leukocyte emigration and vascular permeability in conscious rats. J Appl Physiol (1985). 2000; 89:1561–1568.13. Haslett C, Savill JS, Whyte MK, Stern M, Dransfield I, Meagher LC. Granulocyte apoptosis and the control of inflammation. Philos Trans R Soc Lond B Biol Sci. 1994; 345:327–333.14. Mecklenburgh KI, Walmsley SR, Cowburn AS, Wiesener M, Reed BJ, Upton PD, et al. Involvement of a ferroprotein sensor in hypoxia-mediated inhibition of neutrophil apoptosis. Blood. 2002; 100:3008–3016.15. Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005; 201:105–115.16. Yao L, Kan EM, Lu J, Hao A, Dheen ST, Kaur C, et al. Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia. J Neuroinflammation. 2013; 10:23.17. Kim DH, Billiar TR. Hypoxia activates toll-like receptor 4 signaling in primary mouse hepatocytes through the receptor clustering within lipid rafts. J Korean Surg Soc. 2011; 80:194–203.18. Hyakkoku K, Hamanaka J, Tsuruma K, Shimazawa M, Tanaka H, Uematsu S, et al. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience. 2010; 171:258–267.19. Suzuki Y, Hattori K, Hamanaka J, Murase T, Egashira Y, Mishiro K, et al. Pharmacological inhibition of TLR4-NOX4 signal protects against neuronal death in transient focal ischemia. Sci Rep. 2012; 2:896.20. Jian QC, Wu XW, Song HC, Zheng L. The role of toll like receptor-4 signal pathways activation in ischemia-reperfusion injury of island skin flap. Zhonghua Zheng Xing Wai Ke Za Zhi. 2012; 28:444–448.21. Deitch EA, Sambol JT. The gut-origin hypothesis of MODS. In : Deitch EA, Vincent JL, Windsor A, editors. Sepsis and multiple organ dysfunction. London: Saunders;2002. p. 105–113.22. Partrick DA, Moore FA, Moore EE, Barnett CC Jr, Silliman CC. Neutrophil priming and activation in the pathogenesis of postinjury multiple organ failure. New Horiz. 1996; 4:194–210.23. Biffll WL, Moore EE, Moore FA. The-two-hit model of MODS. In : Deitch EA, Vincent JL, Windsor A, editors. Sepsis and multiple organ dysfunction. London: Saunders;2002. p. 127–132.24. Molloy EJ, O'Neill AJ, Doyle BT, Grantham JJ, Taylor CT, Sheridan-Pereira M, et al. Effects of heat shock and hypoxia on neonatal neutrophil lipopolysaccharide responses: altered apoptosis, Toll-like receptor-4 and CD11b expression compared with adults. Biol Neonate. 2006; 90:34–39.25. Cavaillon JM, Adib-Conquy M, Fitting C, Adrie C, Payen D. Cytokine cascade in sepsis. Scand J Infect Dis. 2003; 35:535–544.26. Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med. 2001; 163:316–321.27. Schmitz K, Jennewein M, Pohlemann T, Seekamp A, Oberringer M. Reoxygenation attenuates the adhesion of neutrophils to microvascular endothelial cells. Angiology. 2011; 62:155–162.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Mild Therapeutic Hypothermia on the non-Vf Cardiac arrest
- Extracellular Vesicles of Neutrophils
- Xylitol Mitigate Neutrophil Inflammatory Response Against Porphyromonas gingivalis Infection
- Exogenous Local Hyperthermia at 41o C Is Effective to Eliminate Mouse Model of Sporotrichosis, Independent of Neutrophil Extracellular Traps Formation
- Targeted temperature management for postcardiac arrest syndrome