Cancer Res Treat.
2017 Apr;49(2):322-329. 10.4143/crt.2016.091.
Blocking Interleukin-4 Receptor α Using Polyethylene Glycol Functionalized Superparamagnetic Iron Oxide Nanocarriers to Inhibit Breast Cancer Cell Proliferation
- Affiliations
-
- 1Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia.
- 2Prince Naif Health Research Center, College of Medicine, King Saud University, Riyadh, Saudi Arabia.
- 3Department of Community Health Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia.
- 4Department of Radiological Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia. achraf.alfaraj@gmail.com
- KMID: 2378104
- DOI: http://doi.org/10.4143/crt.2016.091
Abstract
- PURPOSE
The specific targeting of interleukin-4 receptor α (IL4Rα) receptor offers a promising therapeutic approach for inhibition of tumor cell progression in breast cancer patients. In the current study, the in vitro efficacy of superparamagnetic iron oxide nanoparticles conjugated with anti-IL4Rα blocking antibodies (SPION-IL4Rα) via polyethylene glycol polymers was evaluated in 4T1 breast cancer cells.
MATERIALS AND METHODS
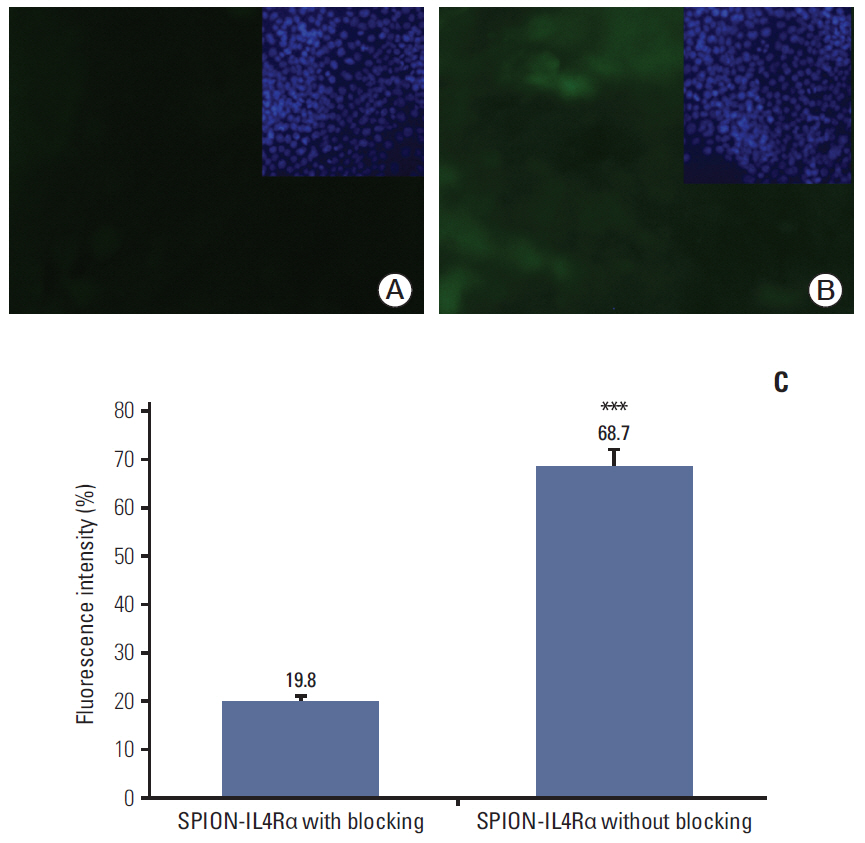
Cell viability, reactive oxygen species generation, and apoptosis frequency were assessed in vitro in 4T1 cancer cell lines following exposure to SPION-IL4Rα alone or combined with doxorubicin. In addition, immunofluorescence assessments and fluorimetrywere performed to confirm the specific targeting and interaction of the developed nanocarriers with IL4Rα receptors in breast cancer cells.
RESULTS
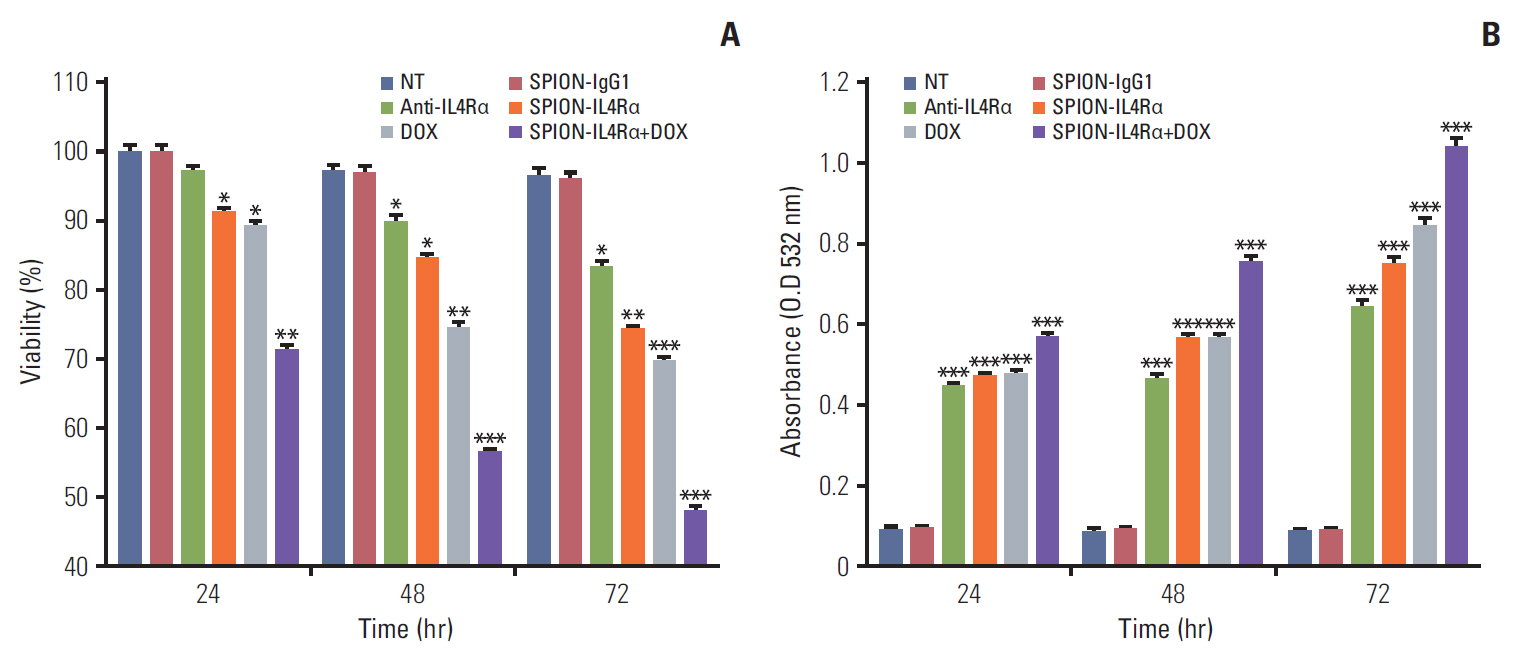
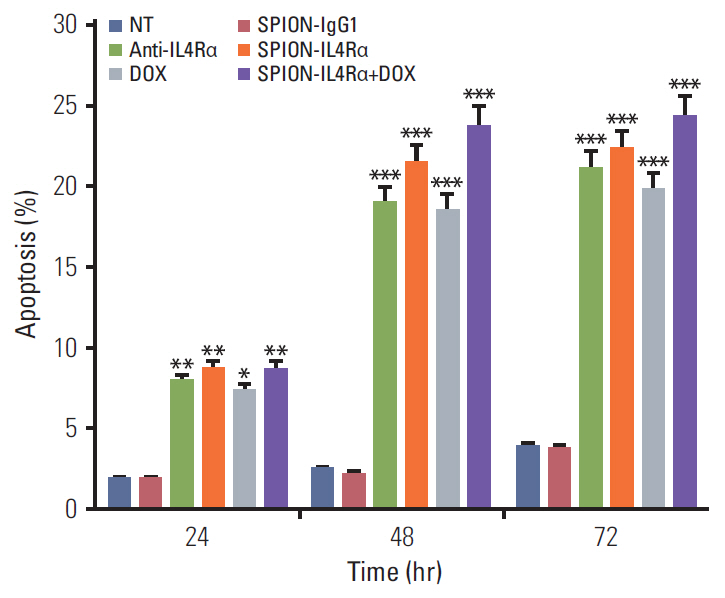
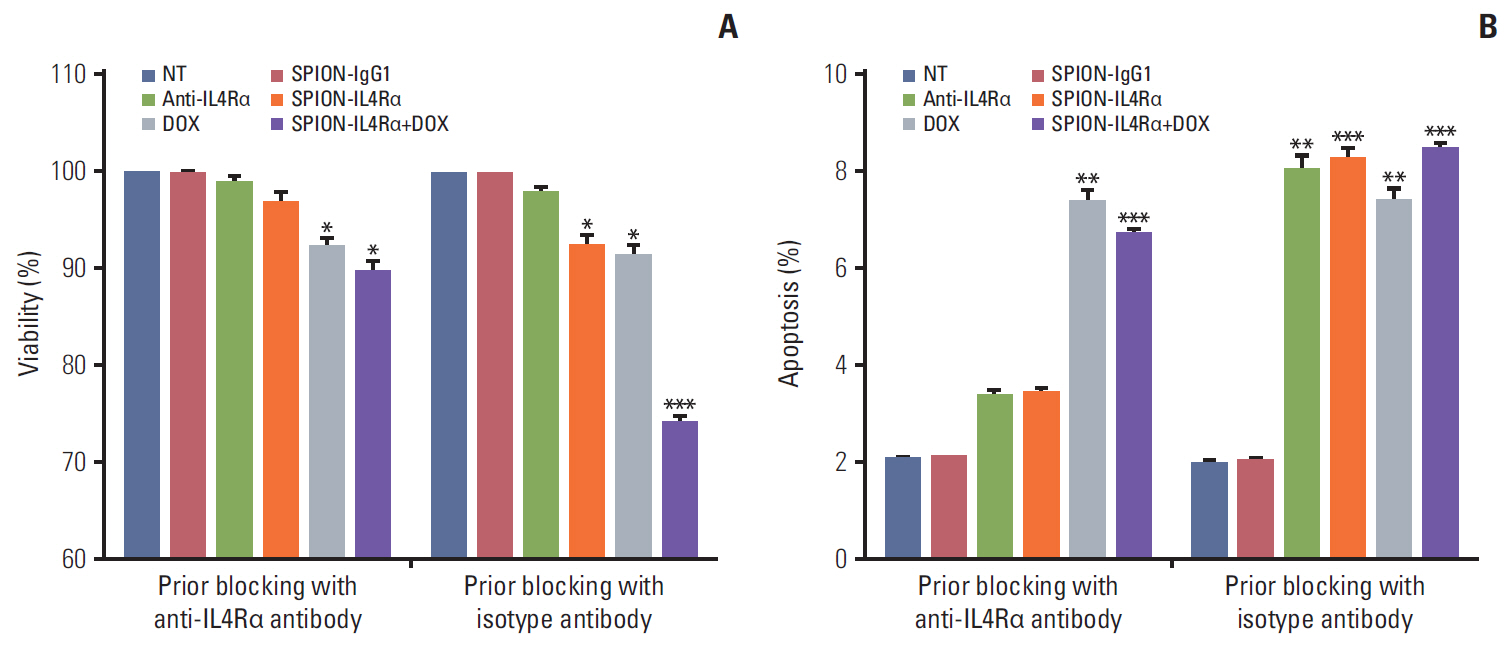
Blocking of IL4Rα receptors caused a significant decrease in cell viability and induced apoptosis in 4T1 cells. In addition, combined treatment with SPION-IL4Rα+doxorubicin caused significant increases in cell death, apoptosis, and oxidative stress compared to either SPION-IL4Rα or doxorubicin alone, indicating the enhanced therapeutic efficacy of this combination. The decrease in fluorescence intensity upon immunofluorescence and fluorimetry assays combined with increased viability and decreased apoptosis following the blocking of IL4Rα receptors confirmed the successful binding of the synthesized nanocarriers to the target sites on murine 4T1 breast cancerous cells.
CONCLUSION
These results suggest that SPION-IL4Rα nanocarriers might be used for successfulreduction of tumor growth and inhibition of progression of metastasis in vivo.
Keyword
MeSH Terms
-
Antibodies, Blocking
Apoptosis
Biomarkers
Breast Neoplasms*
Breast*
Cell Death
Cell Line
Cell Proliferation*
Cell Survival
Doxorubicin
Drug Delivery Systems
Fluorescence
Fluorescent Antibody Technique
Fluorometry
Humans
In Vitro Techniques
Interleukin-4*
Iron*
Nanoparticles
Neoplasm Metastasis
Oxidative Stress
Polyethylene Glycols*
Polyethylene*
Polymers
Reactive Oxygen Species
Antibodies, Blocking
Biomarkers
Doxorubicin
Interleukin-4
Iron
Polyethylene
Polyethylene Glycols
Polymers
Reactive Oxygen Species
Figure
Reference
-
References
1. DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014; 64:252–71.
Article2. Ben-Baruch A. Host microenvironment in breast cancer development: inflammatory cells, cytokines and chemokines in breast cancer progression: reciprocal tumor-microenvironment interactions. Breast Cancer Res. 2003; 5:31–6.
Article3. DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009; 16:91–102.
Article4. Hosoyama T, Aslam MI, Abraham J, Prajapati SI, Nishijo K, Michalek JE, et al. IL-4R drives dedifferentiation, mitogenesis, and metastasis in rhabdomyosarcoma. Clin Cancer Res. 2011; 17:2757–66.
Article5. Joshi BH, Leland P, Lababidi S, Varrichio F, Puri RK. Interleukin- 4 receptor alpha overexpression in human bladder cancer correlates with the pathological grade and stage of the disease. Cancer Med. 2014; 3:1615–28.6. Joshi BH, Leland P, Asher A, Prayson RA, Varricchio F, Puri RK. In situ expression of interleukin-4 (IL-4) receptors in human brain tumors and cytotoxicity of a recombinant IL-4 cytotoxin in primary glioblastoma cell cultures. Cancer Res. 2001; 61:8058–61.7. Al Faraj A, Shaik AS, Al Sayed B, Halwani R, Al Jammaz I. Specific targeting and noninvasive imaging of breast cancer stem cells using single-walled carbon nanotubes as novel multimodality nanoprobes. Nanomedicine (Lond). 2016; 11:31–46.
Article8. Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008; 7:771–82.
Article9. Rosen JE, Chan L, Shieh DB, Gu FX. Iron oxide nanoparticles for targeted cancer imaging and diagnostics. Nanomedicine. 2012; 8:275–90.
Article10. Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P, et al. Drug delivery systems: an updated review. Int J Pharm Investig. 2012; 2:2–11.
Article11. Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013; 65:157–70.
Article12. Al Faraj A, Shaik AP, Shaik AS. Effect of surface coating on the biocompatibility and in vivo MRI detection of iron oxide nanoparticles after intrapulmonary administration. Nanotoxicology. 2015; 9:825–34.13. Al Faraj A, Shaik AS, Afzal S, Al-Muhsen S, Halwani R. Specific targeting and noninvasive magnetic resonance imaging of an asthma biomarker in the lung using polyethylene glycol functionalized magnetic nanocarriers. Contrast Media Mol Imaging. 2016; 11:172–83.
Article14. Yang S, Zhang JJ, Huang XY. Mouse models for tumor metastasis. Methods Mol Biol. 2012; 928:221–8.
Article15. Al Faraj A, Shaik AP, Shaik AS. Magnetic single-walled carbon nanotubes as efficient drug delivery nanocarriers in breast cancer murine model: noninvasive monitoring using diffusion- weighted magnetic resonance imaging as sensitive imaging biomarker. Int J Nanomedicine. 2015; 10:157–68.16. Roca H, Craig MJ, Ying C, Varsos ZS, Czarnieski P, Alva AS, et al. IL-4 induces proliferation in prostate cancer PC3 cells under nutrient-depletion stress through the activation of the JNK-pathway and survivin up-regulation. J Cell Biochem. 2012; 113:1569–80.17. Zhang WJ, Li BH, Yang XZ, Li PD, Yuan Q, Liu XH, et al. IL-4-induced Stat6 activities affect apoptosis and gene expression in breast cancer cells. Cytokine. 2008; 42:39–47.
Article18. Todaro M, Lombardo Y, Francipane MG, Alea MP, Cammareri P, Iovino F, et al. Apoptosis resistance in epithelial tumors is mediated by tumor-cell-derived interleukin-4. Cell Death Differ. 2008; 15:762–72.
Article19. Venmar KT, Carter KJ, Hwang DG, Dozier EA, Fingleton B. IL4 receptor ILR4alpha regulates metastatic colonization by mammary tumors through multiple signaling pathways. Cancer Res. 2014; 74:4329–40.20. Hengartner MO. The biochemistry of apoptosis. Nature. 2000; 407:770–6.
Article21. Hurley LH. DNA and its associated processes as targets for cancer therapy. Nat Rev Cancer. 2002; 2:188–200.
Article22. Roth F, De La Fuente AC, Vella JL, Zoso A, Inverardi L, Serafini P. Aptamer-mediated blockade of IL4Ralpha triggers apoptosis of MDSCs and limits tumor progression. Cancer Res. 2012; 72:1373–83.23. Niu G, Zhu L, Ho DN, Zhang F, Gao H, Quan Q, et al. Longitudinal bioluminescence imaging of the dynamics of doxorubicin induced apoptosis. Theranostics. 2013; 3:190–200.
Article24. Bao L, Haque A, Jackson K, Hazari S, Moroz K, Jetly R, et al. Increased expression of P-glycoprotein is associated with doxorubicin chemoresistance in the metastatic 4T1 breast cancer model. Am J Pathol. 2011; 178:838–52.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cancer -Targeted MR Molecular Imaging
- New Frontiers in Molecular Imaging with Superparamagnetic IronOxide Nanoparticles (SPIONs): Efficacy, Toxicity, and FutureApplications
- Leukocytoclastic Vasulitis Induced by Methoxy Polyethylene Glycol-Epoetin Beta
- Diagnostic value of magnetic resonance imaging using superparamagnetic iron oxide for axillary node metastasis in patients with breast cancer: a meta-analysis
- A Randomized Prospective Trial Comparing a New Polyethylene Glycol Based Lavage Solution with the Standard Polyethylene Glycol Solution in the Preparation of Patients Undergoing Colonoscopy (Clinical trial of new PEG solution in bowel preparation)