Nat Prod Sci.
2016 Dec;22(4):231-237. 10.20307/nps.2016.22.4.231.
A Fruit Extract of Paeonia anomala Attenuates Chronic Alcohol-induced Liver Damage in Rats
- Affiliations
-
- 1Natural Products Research Center, Korea Institute of Science and Technology (KIST) Gangneung Institute, Gangneung, Korea. cwnho@kist.re.kr
- 2Convergence Research Center for Smart Farm Solution, Korea Institute of Science and Technology (KIST) Gangneung Institute, Gangneung, Korea.
- 3Systems Biotechnology Research Center, Korea Institute of Science and Technology (KIST) Gangneung Institute, Gangneung, Korea.
- 4Department of Life Science, Sogang University, Seoul, Korea.
- 5Institute of Chemistry and Chemical Technology, Ulaanbaatar, Mongolia.
- 6Department of Biological Chemistry, Korea University of Science and Technology, Daejeon, Korea.
- KMID: 2366689
- DOI: http://doi.org/10.20307/nps.2016.22.4.231
Abstract
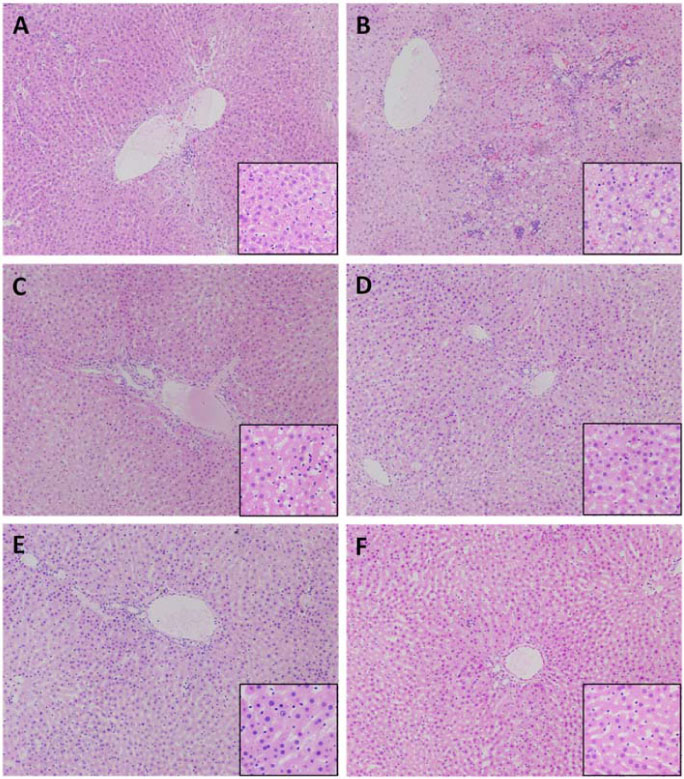
- Prolonged alcohol consumption causes alcoholic liver damage due to the generation of reactive oxygen species, the accumulation of fatty acids, and an increase in inflammatory cytokines in the liver. In this study, the protective effect of a fruit extract of Paeonia anomala (FEPA) against chronic alcohol-induced liver damage was evaluated in Sprague-Dawley rats fed an ethanol or a control Lieber-DeCarli diet for 5 weeks to induce alcoholic liver damage. FEPA (50, 25, and 10 mg/kg body weight/day) as well as the reference control silymarin (25 mg/kg body weight/day) were administered along with the ethanol diet. FEPA protected against increases in alanine aminotransferase and aspartate aminotransferase in serum and attenuated alcohol-induced increases in triglycerides, tumor necrosis factor alpha, thiobarbituric acid-reactive substances, and cytochrome P450 2E1 enzyme activity in the liver compared with the group treated with ethanol only. Anti-oxidative defenses such as the total glutathione level and glutathione peroxidase activity were increased by FEPA treatment. These results suggest that FEPA exerts protective effects against chronic alcohol-induced liver damage by attenuating hepatosteatosis and pro-inflammatory cytokine production and enhancing anti-oxidative defense mechanisms in the liver.
Keyword
MeSH Terms
-
Alanine Transaminase
Alcohol Drinking
Alcoholics
Animals
Aspartate Aminotransferases
Cytochrome P-450 CYP2E1
Cytokines
Defense Mechanisms
Diet
Ethanol
Fatty Acids
Fruit*
Glutathione
Glutathione Peroxidase
Humans
Liver*
Paeonia*
Rats*
Rats, Sprague-Dawley
Reactive Oxygen Species
Silymarin
Triglycerides
Tumor Necrosis Factor-alpha
Alanine Transaminase
Aspartate Aminotransferases
Cytochrome P-450 CYP2E1
Cytokines
Ethanol
Fatty Acids
Glutathione
Glutathione Peroxidase
Reactive Oxygen Species
Silymarin
Triglycerides
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Nagy LE. Ann Rev Nutr. 2004; 24:55–78.2. Stewart S, Jones D, Day CP. Trends Mol Med. 2001; 7:408–413.3. Cederbaum AI, Lu Y, Wu D. Arch Toxicol. 2009; 83:519–548.4. Das SK, Vasudevan DM. Life Sci. 2007; 81:177–187.5. Zima T, Kalousova M. Alcohol Clin Exp Res. 2005; 29:11 Suppl. 110S–115S.6. Wheeler MD, Kono H, Yin M , Nakagami M, Uesugi T, Arteel GE, Gabele E, Rusyn I, Yamashina S, Froh M, Adachi Y, Iimuro Y, Bradford BU, Smutney OM, Connor HD, Mason RP, Goyert SM, Peters JM, Gonzalez FJ, Samulski RJ, Thurman RG. Free Radical Bio Med. 2001; 31:1544–1549.7. Zhou Z, Wang L, Song Z, Lambert JC, McClain CJ, Kang YJ. Am J Path. 2003; 163:1137–1146.8. Zeng T, Xie KQ. Arch Toxicol. 2009; 83:1075–1081.9. Song Z, Deaciuc I, Song M, Lee DY, Liu Y, Ji X, McClain C. Alcohol Clin Exp Res. 2006; 30:407–413.10. de la M Hall P, Lieber CS, DeCarli LM, French SW, Lindros KO, Jarvelainen H, Bode C, Parlesak A, Bode JC. Alcohol Clin xp Res. 2001; 25:254–261.11. Ligaa U, Davaasuren B, Ninjil N. Moscow: Rosselhozakademii;2009.12. Volodya T, Tserenbaljir D, Lkhamjav T. Ulaanbaatar: Admon;2008.13. Oidovsambuu S, Kim CY, Kang K, Dulamjav B, Jigjidsuren T, Nho CW. Planta Med. 2013; 79:116–122.14. Yoo JH, Oidovsambuu S, Kim SM, Jeon NR, Yun JH, Kang K, Jho EH, Lee SB, Nho CW. Food Sci Biotechnol. 2011; 20:127–131.15. Chang TK, Crespi CL, Waxman DJ. Methods Mol Biol. 2006; 320:127–131.16. Lee CS, Ho DV, Chan JY. FEBS J. 2013; 280:3609–3620.
Article17. Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Gastroenterology. 1995; 108:218–224.18. An L, Wang X, Cederbaum AI. Arch Toxicol. 2012; 86:1337–1348.19. Thurman RG. Am J Physiol-Gastr L. 1998; 275:G605–G611.20. Salaspuro MP, Shaw S, Jayatilleke E, Ross WA, Lieber CS. Hepatology. 1981; 1:33–38.21. Day CP. Liver Transplant. 2007; 13:11 Suppl 2. S69–S75.22. Meagher EA, Barry OP, Burke A, Lucey MR, Lawson JA, Rokach J, FitzGerald GA. J Clin Invest. 1999; 104:805–813.23. Sunde RA, Hoekstra WG. Nutr Rev. 1980; 38:265–273.24. Yurt B, Celik I. Food Chem Toxicol. 2011; 49:508–513.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pretreatment of Albino Rats with Methanolic Fruit Extract of Randia Dumetorum (L.) Protects against Alcohol Induced Liver Damage
- Effect of Seeds Extract of Paeonia Lactiflora on Antioxidative System and Lipid Peroxidation of Liver in Rats Fed High-Cholesterol Diet
- The Effect of Extract of Paeonia lactiflora on the Improvement of Ischemic Acute Renal Failure
- Protective effect of an ethanol extract mixture of Aralia elata, Chaenomeles sinensis fruit, and Glycyrrhizae radix against cerebral ischemiareperfusion injury in rats and excitotoxic and oxidative neuronal damage
- A comparative study on the hepatoprotective effect of selenium-nanoparticles and dates flesh extract on carbon tetrachloride induced liver damage in albino rats