Lab Anim Res.
2016 Dec;32(4):231-240. 10.5625/lar.2016.32.4.231.
Characterization the response of Korl:ICR mice to loperamide induced constipation
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources & Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- 2Department of Biomedical Analysis, Korea Bio Polytechnic College, Nonsan, Korea.
- 3Department of Pharmacy, College of Pharmacy, Pusan National University, Busan, Korea.
- 4College of Veterinary Medicine, Kyungpook National University, Daegu, Korea.
- KMID: 2362915
- DOI: http://doi.org/10.5625/lar.2016.32.4.231
Abstract
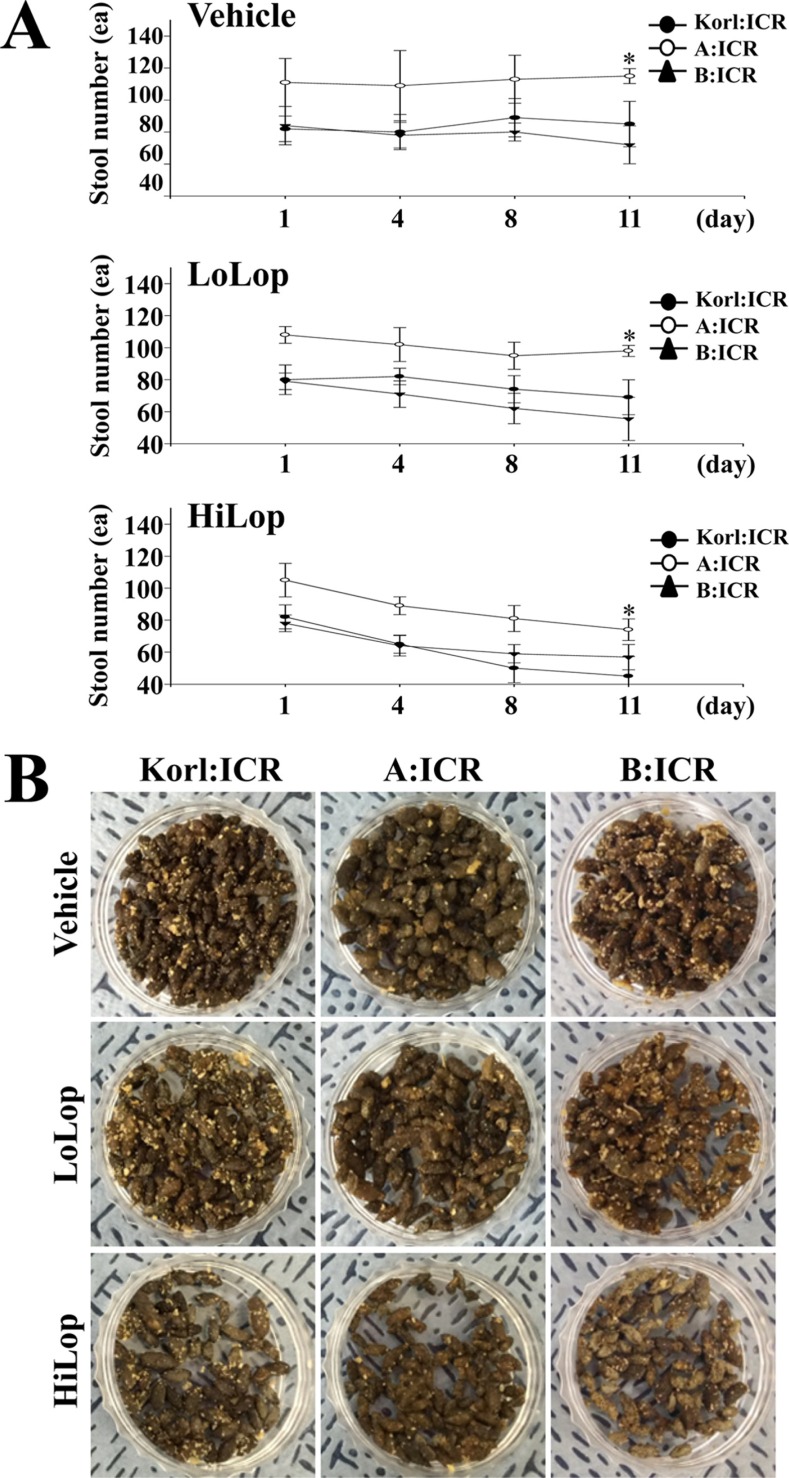
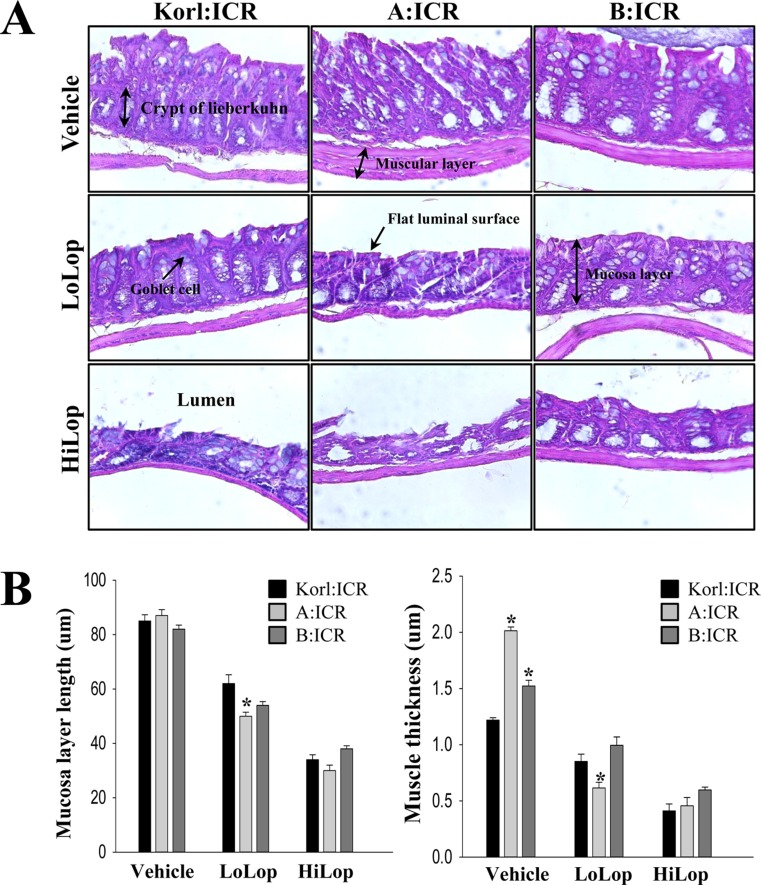
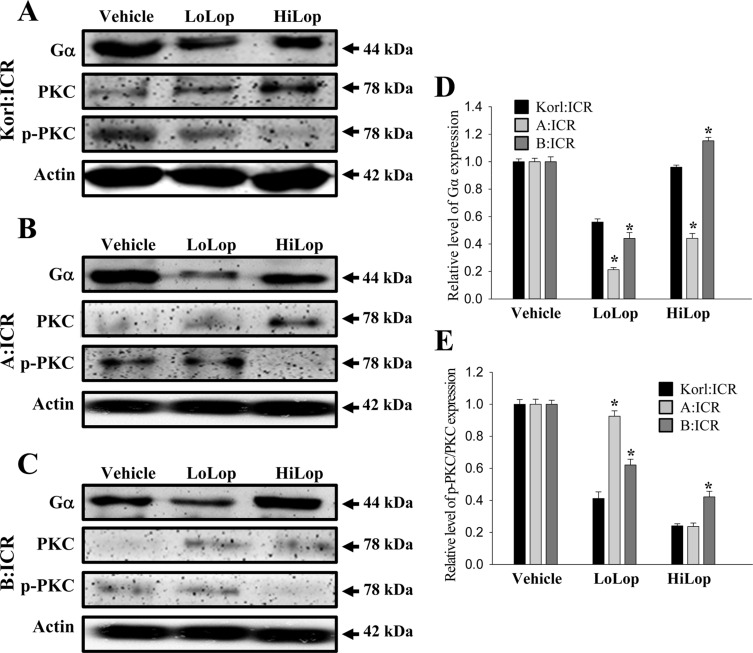
- Animal models of constipation induced with drugs and diet have been widely employed to investigate therapeutic effects and the action mechanism of drugs against this disease. ICR mice were selected to produce this disease model through oral administration of loperamide (Lop), even though SD rats are commonly utilized in studies of constipation. To compare the responses of ICR mice obtained from three different sources to constipation inducers, alterations in stool number, histopathological structure, mucin secretion and opioid-receptor downstream signaling pathway were measured in Korl:ICR (Korea FDA source), A:ICR (USA source) and B:ICR (Japan source) injected with low and high concentrations of Lop (LoLop and HiLop). The number, weight and moisture content of stools decreased significantly in the Lop treated group of all ICR relative to the Vehicle treated group. Additionally, decreased mucosa layer thickness, muscle thickness, and mucin secretion were observed in the transverse colon of Lop treated ICR mice, while a similar number of goblet cells and crypt of lieberkuhn were detected in the same group. Furthermore, a similar change in the level of Gα expression and PKC phosphorylation was detected in the Lop treated group relative to the vehicle treated group, while some differences in the change pattern were observed in the B:ICR group. Therefore, these results of the present study provide strong additional evidence that Korl:ICR, A:ICR and B:ICR derived from different sources have a similar overall response to constipation induced by Lop injection, although there were a few differences in the magnitude of their responses.
MeSH Terms
Figure
Cited by 2 articles
-
Annual tendency of research papers used ICR mice as experimental animals in biomedical research fields
Ji Eun Kim, Jung Hoon Nam, Joon Young Cho, Kil Soo Kim, Dae Youn Hwang
Lab Anim Res. 2017;33(2):171-178. doi: 10.5625/lar.2017.33.2.171.Comparison of laxative effects of fermented soybeans (
Cheonggukjang ) containing toxins and biogenic amines against loperamide-induced constipation mouse model
Ha-Rim Kim, In-Sun Park, Su-Bin Park, Hee-Jong Yang, Do-Youn Jeong, Seon-Young Kim
Nutr Res Pract. 2022;16(4):435-449. doi: 10.4162/nrp.2022.16.4.435.
Reference
-
1. Walia R, Mahajan L, Steffen R. Recent advances in chronic constipation. Curr Opin Pediatr. 2009; 21(5):661–666. PMID: 19606041.
Article2. McCallum IJ, Ong S, Mercer-Jones M. Chronic constipation in adults. BMJ. 2009; 338:b831. PMID: 19304766.
Article3. Emmanuel AV, Tack J, Quigley EM, Talley NJ. Pharmacological management of constipation. Neurogastroenterol Motil. 2009; 21:41–54. PMID: 19824937.
Article4. Leung WK, To KF, Man EP, Chan MW, Hui AJ, Ng SS, Lau JY, Sung JJ. Detection of hypermethylated DNA or cyclooxygenase-2 messenger RNA in fecal samples of patients with colorectal cancer or polyps. Am J Gastroenterol. 2007; 102(5):1070–1076. PMID: 17378912.
Article5. Bharucha AE. Constipation. Best Pract Res Clin Gastroenterol. 2007; 21(4):709–731. PMID: 17643910.
Article6. Nyam DC, Pemberton JH, Ilstrup DM, Rath DM. Long-term results of surgery for chronic constipation. Dis Colon Rectum. 1997; 40(3):273–279. PMID: 9118740.
Article7. Zarate N, Spencer NJ. Chronic constipation: lessons from animal studies. Best Pract Res Clin Gastroenterol. 2011; 25(1):59–71. PMID: 21382579.
Article8. Kim JE, Lee YJ, Kwak MH, Ko J, Hong JT, Hwang DY. Aqueous extracts of Liriope platyphylla induced significant laxative effects on loperamide-induced constipation of SD rats. BMC Complement Altern Med. 2013; 13:333. PMID: 24274470.
Article9. Wintola OA, Sunmonu TO, Afolayan AJ. The effect of Aloe ferox Mill. in the treatment of loperamide-induced constipation in Wistar rats. BMC Gastroenterol. 2010; 10:95. PMID: 20723249.
Article10. Kakino M, Tazawa S, Maruyama H, Tsuruma K, Araki Y, Shimazawa M, Hara H. Laxative effects of agarwood on low-fiber diet-induced constipation in rats. BMC Complement Altern Med. 2010; 10:68. PMID: 21078136.
Article11. Wu D, Wang X, Zhou J, Yuan J, Cui B, An R, Hu Z. Traditional Chinese formula, lubricating gut pill, improves loperamide-induced rat constipation involved in enhance of Cl− secretion across distal colonic epithelium. J Ethnopharmacol. 2010; 130(2):347–353. PMID: 20488235.
Article12. Kojima R, Nozawa K, Doihara H, Keto Y, Kaku H, Yokoyama T, Itou H. Effects of novel TRPA1 receptor agonist ASP7663 in models of drug-induced constipation and visceral pain. Eur J Pharmacol. 2014; 723:288–293. PMID: 24291101.
Article13. Choi JS, Kim JW, Cho HR, Kim KY, Lee JK, Sohn JH, Ku SK. Laxative effects of fermented rice extract in rats with loperamide-induced constipation. Exp Ther Med. 2014; 8(6):1847–1854. PMID: 25371743.
Article14. Chen W, Chung HH, Cheng JT. Opiate-induced constipation related to activation of small intestine opioid μ2-receptors. World J Gastroenterol. 2012; 18(12):1391–1396. PMID: 22493554.
Article15. Zhou M, Jia P, Chen J, Xiu A, Zhao Y, Zhan Y, Chen P, Zhang J. Laxative effects of Salecan on normal and two models of experimental constipated mice. BMC Gastroenterol. 2013; 13:52. PMID: 23514598.
Article16. Li C, Nie SP, Zhu KX, Xiong T, Li C, Gong J, Xie MY. Effect of Lactobacillus plantarum NCU116 on loperamide-induced constipation in mice. Int J Food Sci Nutr. 2015; 66(5):533–538. PMID: 25822005.17. Lee HY, Kim JH, Jeung HW, Lee CU, Kim DS, Li B, Lee GH, Sung MS, Ha KC, Back HI, Kim SY, Park SH, Oh MR, Kim MG, Jeon JY, Im YJ, Hwang MH, So BO, Shin SJ, Yoo WH, Kim HR, Chae HJ, Chae SW. Effects of Ficus carica paste on loperamide-induced constipation in rats. Food Chem Toxicol. 2012; 50:895–902. PMID: 22178225.18. Ono H, Nakamura A, Matsumoto K, Horie S, Sakaguchi G, Kanemasa T. Circular muscle contraction in the mice rectum plays a key role in morphine-induced constipation. Neurogastroenterol Motil. 2014; 26(10):1396–1407. PMID: 25041353.
Article19. Mori T, Shibasaki Y, Matsumoto K, Shibasaki M, Hasegawa M, Wang E, Masukawa D, Yoshizawa K, Horie S, Suzuki T. Mechanisms that underlie μ-opioid receptor agonist-induced constipation: differential involvement of μ-opioid receptor sites and responsible regions. J Pharmacol Exp Ther. 2013; 347(1):91–99. PMID: 23902939.
Article20. Chukhin E, Takala P, Hakko H, Raidma M, Putkonen H, Räsänen P, Terevnikov V, Stenberg JH, Eronen M, Joffe G. In a randomized placebo-controlled add-on study orlistat significantly reduced clozapine-induced constipation. Int Clin Psychopharmacol. 2013; 28(2):67–70. PMID: 23187856.
Article21. Zhao X, Suo HY, Qian Y, Li GJ, Liu ZH, Li J. Therapeutic effects of Lactobacillus casei Qian treatment in activated carbon-induced constipated mice. Mol Med Rep. 2015; 12(2):3191–3199. PMID: 25955533.22. Suo H, Zhao X, Qian Y, Li G, Liu Z, Xie J, Li J. Therapeutic effect of activated carbon-induced constipation mice with Lactobacillus fermentum Suo on treatment. Int J Mol Sci. 2014; 15(12):21875–21895. PMID: 25464378.23. Chia R, Achilli F, Festing MF, Fisher EM. The origins and uses of mouse outbred stocks. Nat Genet. 2005; 37(11):1181–1186. PMID: 16254564.
Article24. Cui S, Chesson C, Hope R. Genetic variation within and between strains of outbred Swiss mice. Lab Anim. 1993; 27(2):116–123. PMID: 8501892.
Article25. Lehoczky JA, Cai WW, Douglas JA, Moran JL, Beier DR, Innis JW. Description and genetic mapping of Polypodia: an X-linked dominant mouse mutant with ectopic caudal limbs and other malformations. Mamm Genome. 2006; 17(9):903–913. PMID: 16964440.
Article26. Holzer P. Opioid receptors in the gastrointestinal tract. Regul Pept. 2009; 155(1-3):11–17. PMID: 19345246.
Article27. Castiglione F, Mantile F, De Berardinis P, Prisco A. How the interval between prime and boost injection affects the immune response in a computational model of the immune system. Comput Math Methods Med. 2012; 2012:842329. PMID: 22997539.
Article28. Matsuda M. Comparison of the incidence of 5-azacytidine-induced exencephaly between MT/HokIdr and Slc:ICR mice. Teratology. 1990; 41(2):147–154. PMID: 1690922.
Article29. Kehrer JP, DiGiovanni J. Comparison of lung injury induced in 4 strains of mice by butylated hydroxytoluene. Toxicol Lett. 1990; 52(1):55–61. PMID: 2356571.
Article30. Carlson GP. Comparison of mouse strains for susceptibility to styrene-induced hepatotoxicity and pneumotoxicity. J Toxicol Environ Health. 1997; 51(2):177–187. PMID: 9176557.
Article31. Livne E, Laufer D, Blumenfeld I. Comparison of in vitro response to growth hormone by chondrocytes from mandibular condyle cartilage of young and old mice. Calcif Tissue Int. 1997; 61(1):62–67. PMID: 9192516.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of commonly used ICR stocks and the characterization of Korl:ICR
- Comparison of therapeutic responses to an anticancer drug in three stocks of ICR mice derived from three different sources
- Comparison of the response using ICR mice derived from three different sources to multiple low-dose streptozotocin-induced diabetes mellitus
- The Effect of Probiotic on Constipation in Rats
- Comparison of the response using ICR mice derived from three different sources to ethanol/hydrochloric acid-induced gastric injury