Lab Anim Res.
2017 Jun;33(2):187-194. 10.5625/lar.2017.33.2.187.
Comparison of therapeutic responses to an anticancer drug in three stocks of ICR mice derived from three different sources
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources & Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang 627-706, Korea. dyhwang@pusan.ac.kr
- 2Department of Pharmacy, College of Pharmacy, Pusan National University, Busan 46241, Korea.
- 3College of Veterinary Medicine, Kungpook National University, Daegu, 702-701, Korea.
- KMID: 2407434
- DOI: http://doi.org/10.5625/lar.2017.33.2.187
Abstract
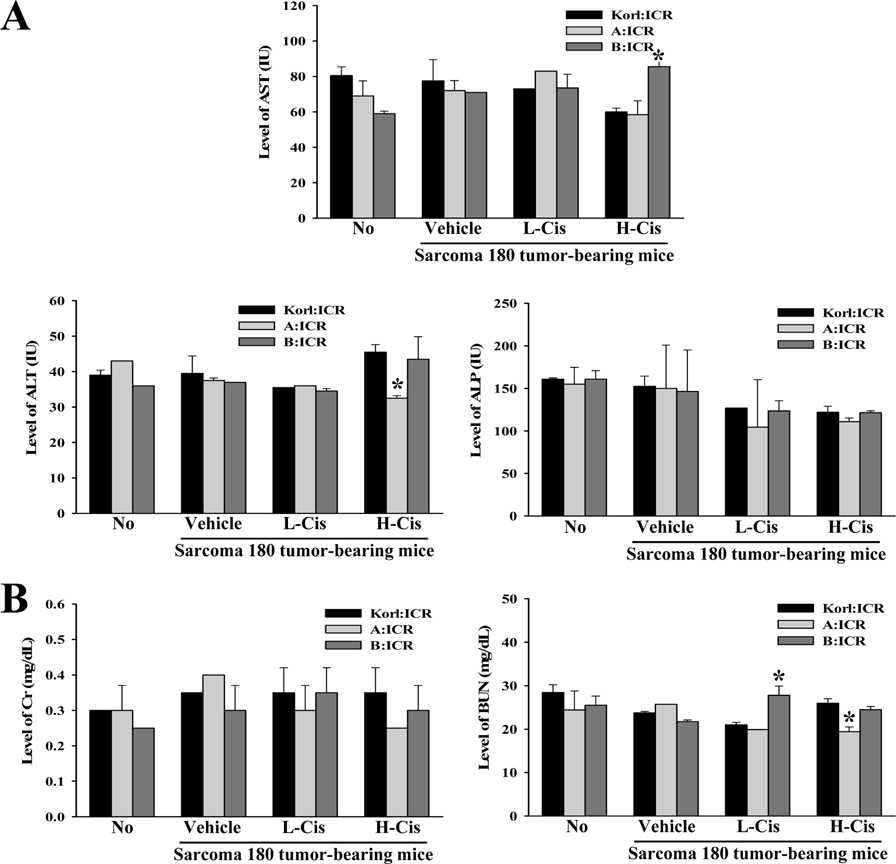
- Korl:ICR mice, established by the Korean National Institute of Food and Drug Safety Evaluation (NIFDS), are characterized based on their genetic variation, response to gastric injury, and response to constipation inducers. To compare the inhibitory responses of ICR stocks obtained from three different sources to the anticancer drug cisplatin (Cis), alterations in tumor volume, histopathological structure, and toxicity were examined in Sarcoma 180 tumor-bearing Korl:ICR, A:ICR (USA source), and B:ICR (Japan source) mice treated with low and high concentrations of Cis (L-Cis and H-Cis, respectively). Tumor size and volume were lower in H-Cis-treated mice than in L-Cis-treated mice in all three ICR stocks with no significant differences among stocks. There was a significant enhancement of the necrotizing areas in the histological structures in the L-Cis- and H-Cis-treated groups relative to that in the untreated group. The necrotizing area changes were similar in the Sarcoma 180 tumor-bearing Korl:ICR, A:ICR, and B:ICR mice. However, there were stock-bases differences in the serum biomarkers for liver and kidney toxic effects. In particular, the levels of AST, ALT and BUN increased differently in the three H-Cis-treated ICR stocks, whereas the levels of ALP and CRE were constant. Taken together, the results of the present study indicate that Korl:ICR, A:ICR, and B:ICR mice have similar overall inhibitory responses following Cis treatment of Sarcoma 180-derived solid tumors, although there were some differences in the magnitude of the toxic effects in the three ICR stocks.
Keyword
MeSH Terms
Figure
Reference
-
1. Rice MC, O'Brien SJ. Genetic variance of laboratory outbred Swiss mice. Nature. 1980; 283(5743):157–161.2. Cui S, Chesson C, Hope R. Genetic variation within and between strains of outbred Swiss mice. Lab Anim. 1993; 27(2):116–123.3. Chia R, Achilli F, Festing MF, Fisher EM. The origins and uses of mouse outbred stocks. Nat Genet. 2005; 37(11):1181–1186.4. O'Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009; 14(5):511–522.5. Zhong SZ, Ge QH, Qu R, Li Q, Ma SP. Paeonol attenuates neurotoxicity and ameliorates cognitive impairment induced by d-galactose in ICR mice. J Neurol Sci. 2009; 277(1-2):58–64.6. Lynch CJ. The so-called Swiss mouse. Lab Anim Care. 1969; 19(2):214–220.7. Snell GD. Biology of the Laboratory Mouse. 1st ed. New York: McGraw-Hill;1941. p. 1–497.8. Hwang SJ, Cho YM, Shin HJ, Kim HD, Choi KM, Kwon KC, Lee SH, Shin HJ, Shin HD, Chung MW. Stratification of outbred ICR mice stocks by genetic variation. Acad J Biotechnol. 2016; 4(11):365–371.9. Song SH, Kim JE, Go J, Koh EK, Sung JE, Lee HA, Choi KM, Kim HD, Jung YS, Kim KS, Hwang DY. Comparison of the response using ICR mice derived from three different sources to ethanol/hydrochloric acid-induced gastric injury. Lab Anim Res. 2016; 32(1):56–64.10. Kim JE, Yun WB, Sung JE, Lee HA, Choi JY, Choi YS, Jung YS, Kim KS, Hwang DY. Characterization the response of Korl:ICR mice to loperamide induced constipation. Lab Anim Res. 2016; 32(4):231–240.11. Wu HT, Lu FH, Su YC, Ou HY, Hung HC, Wu JS, Yang YC, Chang CJ. In vivo and in vitro anti-tumor effects of fungal extracts. Molecules. 2014; 19(2):2546–2556.12. da Silva, Santos NA, Carvalho Rodrigues MA, Rodrigues JL, Barbosa Junior F, Santos AC. Effect of diabetes on biodistribution, nephrotoxicity and antitumor activity of cisplatin in mice. Chem Biol Interact. 2015; 229:119–131.13. Frank R, Hargreaves R. Clinical biomarkers in drug discovery and development. Nat Rev Drug Discov. 2003; 2(7):566–580.14. Bueters T, Ploeger BA, Visser SA. The virtue of translational PKPD modeling in drug discovery: selecting the right clinical candidate while sparing animal lives. Drug Discov Today. 2013; 18(17-18):853–862.15. Fan J, de Lannoy IA. Pharmacokinetics. Biochem Pharmacol. 2014; 87(1):93–120.16. Kimura T. Natural products and biological activity of the pharmacologically active cauliflower mushroom Sparassis crispa. Biomed Res Int. 2013; 2013:982317.17. Hashi M, Takeshita T. The host-mediated anti-tumor effect of 4-O-methylgluccuronoxylan. Agric Biol Chem. 1979; 43(5):961–967.18. Stefanova TH, Nikolova NJ, Toshkova RA, Neychev HO. Antitumor and immunomodulatory effect of coumarin and 7-hydroxycoumarin against Sarcoma 180 in mice. J Exp Ther Oncol. 2007; 6(2):107–115.19. Snell GD, Russell E, Fekete E, Smith P. Resistance of various inbred strains of mice to tumor homoiotransplants, and its relation to the H-2 allele which each carries. J Natl Cancer Inst. 1953; 14(3):485–491.20. Strong LC. Indications of tissue specificity in a transplantable sarcoma. J Exptl Med. 1924; 39(3):447–456.21. Bradner WT, Pindell MH. Strain specificity of stimulated regression of sarcoma 180. Cancer Res. 1965; 25(6):859–864.22. Diller IC, Donnelly AJ, Fisher ME. Isolation of pleomorphic, acid-fast organisms from serveral strains of mice. Cancer Res. 1967; 27(8):1402–1408.23. Vikis HG, Jackson EN, Krupnick AS, Franklin A, Gelman AE, Chen Q, Piwnica-Worms D, You M. Strain-specific susceptibility for pulmonary metastasis of sarcoma 180 cells in inbred mice. Cancer Res. 2010; 70(12):4859–4867.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the virulence of Streptococcus pneumoniae in ICR mouse stocks of three different origins
- Comparison of the response using ICR mice derived from three different sources to multiple low-dose streptozotocin-induced diabetes mellitus
- Comparison of commonly used ICR stocks and the characterization of Korl:ICR
- Comparison of the response using ICR mice derived from three different sources to ethanol/hydrochloric acid-induced gastric injury
- Comparison of scopolamine-induced cognitive impairment responses in three different ICR stocks