Lab Anim Res.
2017 Jun;33(2):150-156. 10.5625/lar.2017.33.2.150.
Comparison of the response using ICR mice derived from three different sources to multiple low-dose streptozotocin-induced diabetes mellitus
- Affiliations
-
- 1College of Veterinary Medicine, Kyungpook National University, Daegu 41566, Korea. kskim728@knu.ac.kr
- 2Department of Pharmacy, College of Pharmacy, Pusan National University, Busan 46241, Korea.
- 3Department of Biomaterials Science, College of Natural Resources & Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang 50463, Korea.
- KMID: 2407429
- DOI: http://doi.org/10.5625/lar.2017.33.2.150
Abstract
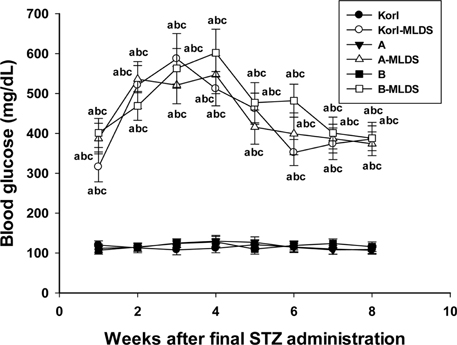
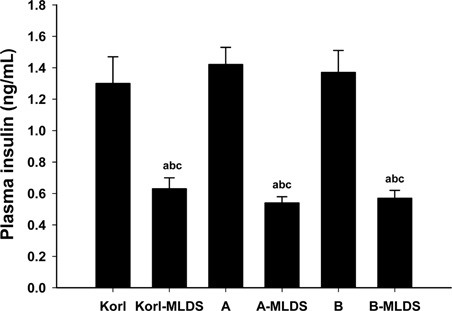
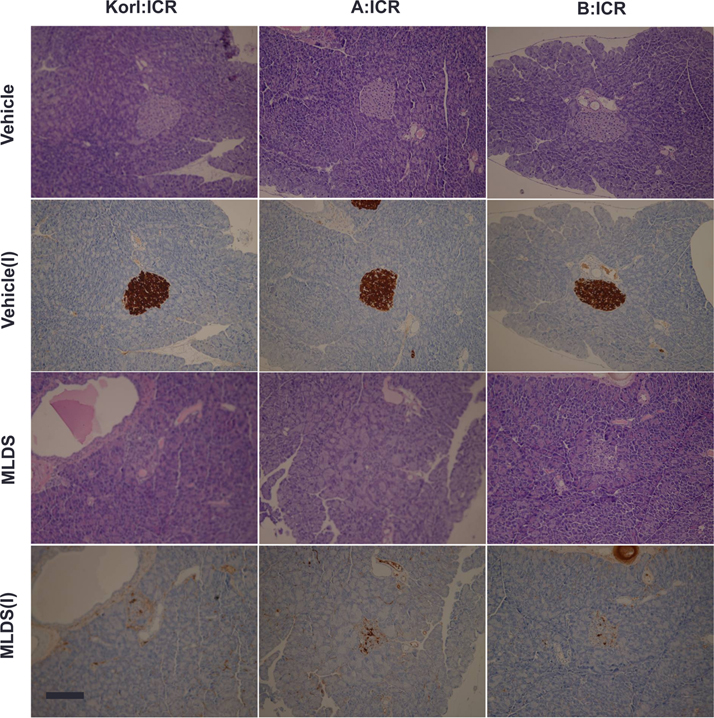
- This study was conducted to compare the multiple low-dose streptozotocin (MLDS)-induced diabetic patterns of Korl:ICR, A:ICR, and B:ICR mice obtained from three different sources. Six-week-old male ICR mice were obtained from three difference sources. Korl:ICR mice were kindly provided by the National Institute of Food and Drug Safety Evaluation (NIFDS). The other two groups of ICR mice were purchased from different vendors located in the United States (A:ICR) and Japan (B:ICR). All ICR mice that received MLDS exhibited overt diabetic symptoms throughout the study, and the incidence and development of diabetes mellitus were similar among the three ICR groups. The diabetic mice exhibited hyperglycemia, loss of body weight gain, decreased plasma insulin levels, impaired glucose tolerance, decreased number of insulin-positive cells, and decreased size of β-cells in the pancreas. The diabetes symptoms increased as the blood glucose level increased in the three ICR groups. In particular, the level of blood glucose in the STZ-treated group was higher in Korl:ICR and A:ICR mice than in B:ICR mice. The plasma insulin levels, glucose tolerance, blood chemistry, and morphological appearance of the pancreas were very similar in the ICR groups obtained from the three different sources. In conclusion, our results suggest that Korl:ICR, A:ICR, and B:ICR mice from different sources had similar overall responses to multiple low-dose STZ to induce diabetes mellitus.
MeSH Terms
Figure
Reference
-
1. Chia R, Achilli F, Festing MF, Fisher EM. The origins and uses of mouse outbred stocks. Nat Genet. 2005; 37(11):1181–1186.2. Hubrecht RC, Kirkwood J. The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals. 8th ed. Oxford: Wiley-Blackwell;2010.3. Vallender EJ, Miller GM. Nonhuman primate models in the genomic era: a paradigm shift. ILAR J. 2013; 54(2):154–165.4. Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory Animal Medicine. 2nd ed. New York: Academic Press;2002.5. Cui S, Chesson C, Hope R. Genetic variation within and between strains of outbred Swiss mice. Lab Anim. 1993; 27(2):116–123.6. Lehoczky JA, Cai WW, Douglas JA, Moran JL, Beier DR, Innis JW. Description and genetic mapping of Polypodia: an X-linked dominant mouse mutant with ectopic caudal limbs and other malformations. Mamm Genome. 2006; 17(9):903–913.7. Al-Awar A, Kupai K, Veszelka M, Szûcs G, Attieh Z, Murlasits Z, Török S, Pósa A, Varga C. Experimental Diabetes Mellitus in Different Animal Models. J Diabetes Res. 2016; 2016:9051426.8. Horton ES. NIDDM--the devastating disease. Diabetes Res Clin Pract. 1995; 28:Suppl. S3–S11.9. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004; 27(5):1047–1053.10. Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011; 378(9785):31–40.11. Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008; 51(2):216–226.12. Skovsø S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Investig. 2014; 5(4):349–358.13. Sakata N, Yoshimatsu G, Tsuchiya H, Egawa S, Unno M. Animal models of diabetes mellitus for islet transplantation. Exp Diabetes Res. 2012; 2012:256707.14. Tesch GH, Nikolic-Paterson DJ. Recent insights into experimental mouse models of diabetic nephropathy. Nephron Exp Nephrol. 2006; 104(2):e57–e62.15. Müller A, Schott-Ohly P, Dohle C, Gleichmann H. Differential regulation of Th1-type and Th2-type cytokine profiles in pancreatic islets of C57BL/6 and BALB/c mice by multiple low doses of streptozotocin. Immunobiology. 2002; 205(1):35–50.16. Brentjens R, Saltz L. Islet cell tumors of the pancreas: the medical oncologist's perspective. Surg Clin North Am. 2001; 81(3):527–542.17. Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992; 326(8):519–523.18. Dominguez S, Denys A, Madeira I, Hammel P, Vilgrain V, Menu Y, Bernades P, Ruszniewski P. Hepatic arterial chemoembolization with streptozotocin in patients with metastatic digestive endocrine tumours. Eur J Gastroenterol Hepatol. 2000; 12(2):151–157.19. Yang SJ, Je Lee W, Kim EA, Dal Nam K, Hahn HG, Young Choi S, Cho SW. Effects of N-adamantyl-4-methylthiazol-2-amine on hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2014; 736:26–34.20. Liu Y, Sun J, Rao S, Su Y, Li J, Li C, Xu S, Yang Y. Antidiabetic activity of mycelia selenium-polysaccharide from Catathelasma ventricosum in STZ-induced diabetic mice. Food Chem Toxicol. 2013; 62:285–291.21. Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001; 50(6):537–546.22. Konrad RJ, Kudlow JE. The role of O-linked protein glycosylation in beta-cell dysfunction. Int J Mol Med. 2002; 10(5):535–539.23. Chen V, Ianuzzo CD. Dosage effect of streptozotocin on rat tissue enzyme activities and glycogen concentration. Can J Physiol Pharmacol. 1982; 60(10):1251–1256.24. Herold KC, Vezys V, Sun Q, Viktora D, Seung E, Reiner S, Brown DR. Regulation of cytokine production during development of autoimmune diabetes induced with multiple low doses of streptozotocin. J Immunol. 1996; 156(9):3521–3527.25. Ogawa J, Takahashi S, Fujiwara T, Fukushige J, Hosokawa T, Izumi T, Kurakata S, Horikoshi H. Troglitazone can prevent development of type 1 diabetes induced by multiple low-dose streptozotocin in mice. Life Sci. 1999; 65(12):1287–1296.26. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007; 87(4):1409–1439.27. Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005; 172(3):367–379.28. Harris EH. Elevated liver function tests in type 2 diabetes. Clin Diabetes. 2005; 23(3):115–119.29. Liu Y, Sun J, Rao S, Su Y, Yang Y. Antihyperglycemic, antihyperlipidemic and antioxidant activities of polysaccharides from Catathelasma ventricosum in streptozotocin-induced diabetic mice. Food Chem Toxicol. 2013; 57:39–45.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of combined treatment of nicotinamide with insulin on multiple low dose streptozotocin induced diabetic mice
- Comparison of the response using ICR mice derived from three different sources to ethanol/hydrochloric acid-induced gastric injury
- Comparison of therapeutic responses to an anticancer drug in three stocks of ICR mice derived from three different sources
- Effects of Diabetes Mellitus on the Disposition of Tofacitinib, a Janus Kinase Inhibitor, in Rats
- The protective effect of Amomum xanthoides extract against alloxan-induced diabetes through the suppression of NFkappaB activation