Lab Anim Res.
2016 Mar;32(1):56-64. 10.5625/lar.2016.32.1.56.
Comparison of the response using ICR mice derived from three different sources to ethanol/hydrochloric acid-induced gastric injury
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources & Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang 627-706, Korea. dyhwang@pusan.ac.kr
- 2Laboratory Animal Resources Division, Toxicological Evaluation Research Department, National Institute of Food and Drug Safety Evaluation, Cheongju 28159, Korea.
- 3Department of Pharmacy, College of Pharmacy, Pusan National University, Busan 46241, Korea.
- 4College of Veterinary Medicine, Kyungpook National University, Daegu 41566, Korea.
- KMID: 2312154
- DOI: http://doi.org/10.5625/lar.2016.32.1.56
Abstract
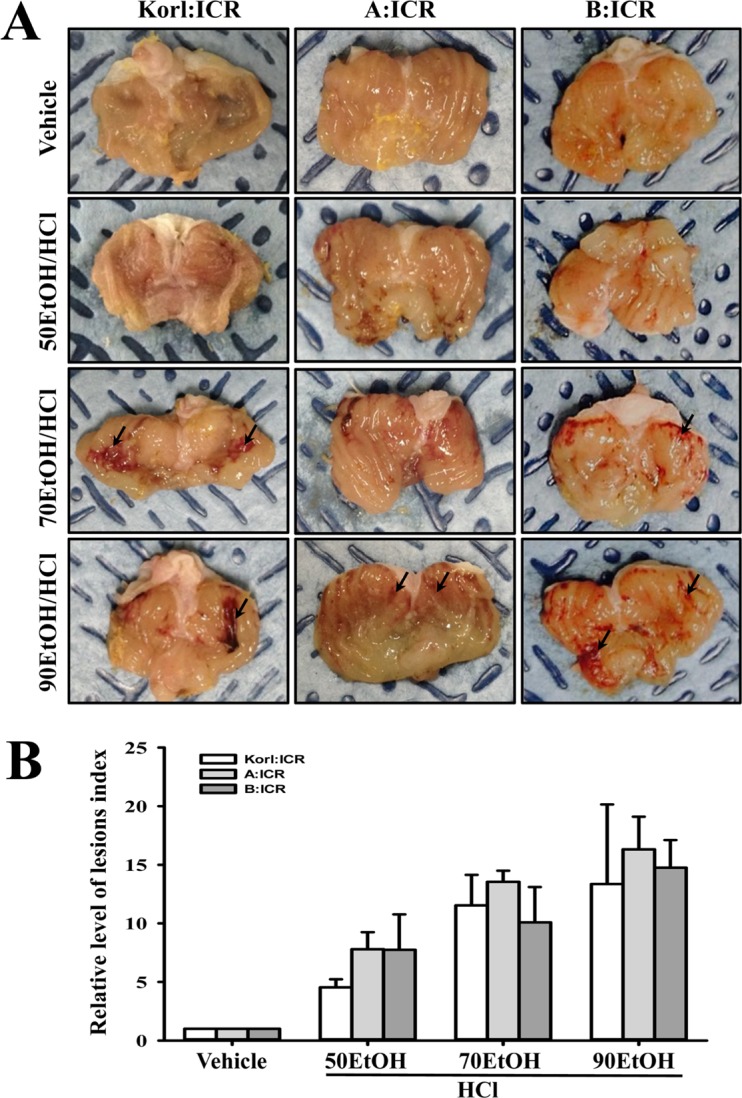
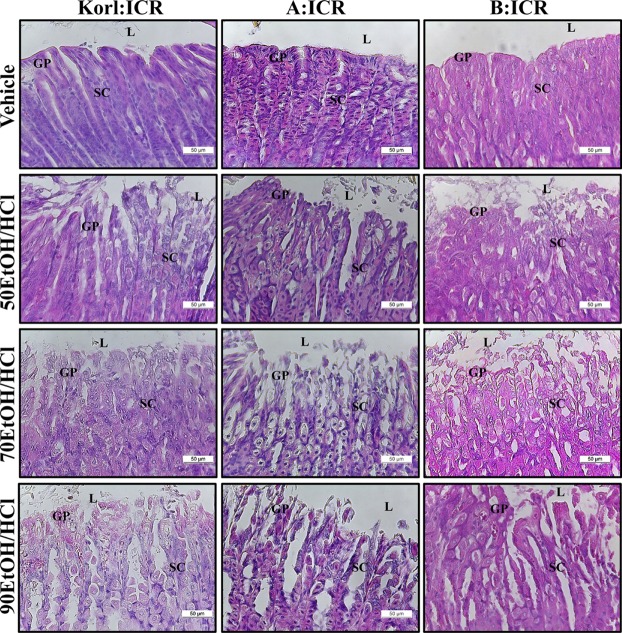
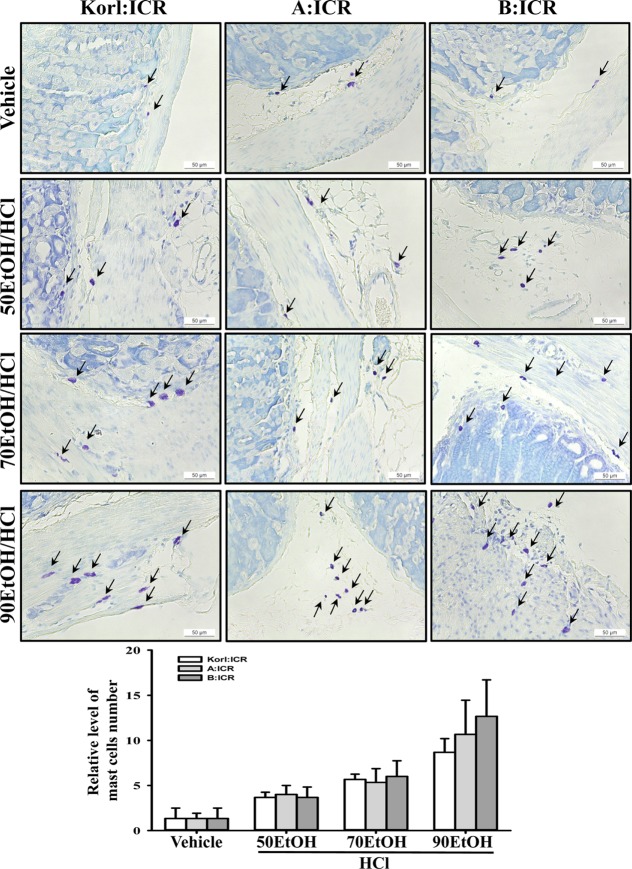
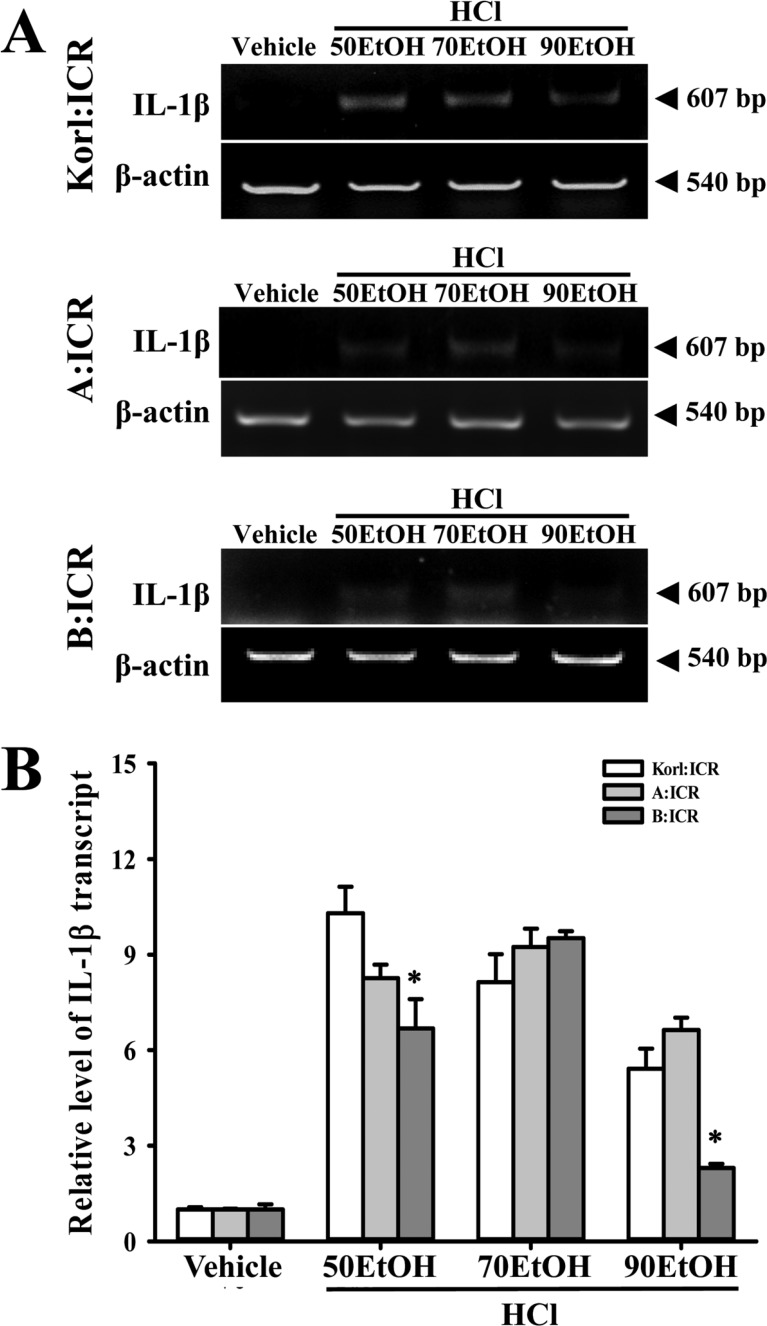
- Animal models for gastric ulcers produced by physical, pharmacological and surgical methods have been widely employed to evaluate therapeutic drugs and investigate the mechanism of action of this disease. ICR mice were selected to produce this model, even though several mice and rats have been widely used in studies of gastric ulcers. To compare the responses of ICR mice obtained from three different sources to gastric ulcer inducers, alterations in gastric injury, histopathological structure, and inflammation were measured in Korl:ICR (Korea NIFDS source), A:ICR (USA source) and B:ICR (Japan source) treated with three concentrations of ethanol (EtOH) (50, 70, and 90%) in 150 mM hydrochloric acid (HCl) solution. Firstly, the stomach lesion index gradually increased as the EtOH concentration increased in three ICR groups. Moreover, a significant increase in the level of mucosal injury, edema and the number of inflammatory cells was similarly detected in the EtOH/HCl treated group compared with the vehicle treated group in three ICR groups. Furthermore, the number of infiltrated mast cells and IL-1β expression were very similar in the ICR group derived from three different sources, although some differences in IL-1β expression were detected. Especially, the level of IL-1β mRNA in 50 and 90EtOH/HCl treated group was higher in Korl:ICR and A:ICR than B:ICR. Overall, the results of this study suggest that Korl:ICR, A:ICR and B:ICR derived from different sources have an overall similar response to gastric ulcer induced by EtOH/HCl administration, although there were some differences in the magnitude of their responses.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Annual tendency of research papers used ICR mice as experimental animals in biomedical research fields
Ji Eun Kim, Jung Hoon Nam, Joon Young Cho, Kil Soo Kim, Dae Youn Hwang
Lab Anim Res. 2017;33(2):171-178. doi: 10.5625/lar.2017.33.2.171.Comparison of commonly used ICR stocks and the characterization of Korl:ICR
Hye-Jun Shin, Young Min Cho, Hee Jung Shin, Hae Deun Kim, Kyung Min Choi, Mi Gyeong Kim, Hyoung Doo Shin, Myeon-Woo Chung
Lab Anim Res. 2017;33(1):8-14. doi: 10.5625/lar.2017.33.1.8.
Reference
-
1. Viana AF, Fernandes HB, Silva FV, Oliveira IS, Freitas FF, Machado FD, Costa CL, Arcanjo DD, Chaves MH, Oliveira FA, Oliveira RC. Gastroprotective activity of Cenostigma macrophyllum Tul. var acuminata Teles Freire leaves on experimental ulcer models. J Ethnopharmacol. 2013; 150(1):316–323. PMID: 24035848.2. Yamamoto S, Watabe K, Araki H, Kamada Y, Kato M, Kizu T, Kiso S, Tsutsui S, Tsujii M, Kihara S, Funahashi T, Shimomura I, Hayashi N, Takehara T. Protective role of adiponectin against ethanol-induced gastric injury in mice. Am J Physiol Gastrointest Liver Physiol. 2012; 302(8):G773–G780. PMID: 22323129.
Article3. Wang J, Zhang T, Zhu L, Ma C, Wang S. Anti-ulcerogenic effect of Zuojin Pill against ethanol-induced acute gastric lesion in animal models. J Ethnopharmacol. 2015; 173:459–467. PMID: 25959443.
Article4. Chen S, Zhu K, Wang R, Zhao X. Preventive effect of polysaccharides from the large yellow croaker swim bladder on HCl/ethanol induced gastric injury in mice. Exp Ther Med. 2014; 8(1):316–322. PMID: 24944640.
Article5. Yang Y, Lee J, Rhee MH, Yu T, Baek KS, Sung NY, Kim Y, Yoon K, Kim JH, Kwak YS, Hong S, Kim JH, Cho JY. Molecular mechanism of protopanaxadiol saponin fraction-mediated anti-inflammatory actions. J Ginseng Res. 2015; 39(1):61–68. PMID: 25535478.
Article6. Byun JS. Protective effects of Coptidis Rhizoma on ethanol-induced gastric ulcer in mice. Korean J Orient Physiol Pathol. 2012; 26(1):67–73.7. Kim JS, Lim SW. The effects of Sasammaickmoondong-tang against gastric mucosal lesions. J Korean Orient Med. 2003; 24(2):121–137.8. Adinortey MB, Ansah C, Galyuon I, Nyarko A. In Vivo models used for evaluation of potential antigastroduodenal ulcer agents. Ulcers. 2013; 2013:12.9. Brzozowski T, Konturek PC, Konturek SJ, Kwiecién S, Pajdo R, Brzozowska I, Hahn EG. Involvement of endogenous cholecystokinin and somatostatin in gastroprotection induced by intraduodenal fat. J Clin Gastroenterol. 1998; 27(Suppl 1):S125–S137. PMID: 9872509.
Article10. Nam DE, Kim OK, Shim TJ, Lee JK, Hwang KT. Inhibitory effects of Chios Mastic Gum on gastric acid secretion by histamine-related pathway in a rat model and primary parietal cells. J Korean Soc Food Sci Nutr. 2014; 43(10):1500–1509.
Article11. Kazumori H, Ishihara S, Fukuda R, Kinoshita Y. Time-course changes of ECL cell markers in acetic acid-induced gastric ulcers in rats. Aliment Pharmacol Ther. 2002; 16(2):10–19. PMID: 11966519.
Article12. Liu J, Wang F, Luo H, Liu A, Li K, Li C, Jiang Y. Protective effect of butyrate against ethanol-induced gastric ulcers in mice by promoting the anti-inflammatory, anti-oxidant and mucosal defense mechanisms. Int Immunopharmacol. 2016; 30:179–187. PMID: 26604089.
Article13. Robert A, Nezamis JE, Lancaster C, Hanchar AJ. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology. 1979; 77(3):433–443. PMID: 456839.14. Oates PJ, Hakkinen JP. Studies on the mechanism of ethanol-induced gastric damage in rats. Gastroenterology. 1998; 94(1):10–21. PMID: 3335281.
Article15. Ku SK, Seo BI, Park JH, Park GY, Seo YB, Kim JS, Lee HS, Roh SS. Effect of Lonicerae Flos extracts on reflux esophagitis with antioxidant activity. World J Gastroenterol. 2009; 15(38):4799–4805. PMID: 19824114.16. Laine L, Weinstein WM. Histology of alcoholic hemorrhagic "gastritis": a prospective evaluation. Gastroenterology. 1988; 94(6):1254–1262. PMID: 3258836.
Article17. Prussin C, Metcalfe DD. 4. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2003; 111(2 suppl):S486–S494. PMID: 12592295.
Article18. Anderson DC, Kodukula K. Biomarkers in pharmacology and drug discovery. Biochem Pharmacol. 2014; 87(1):172–188. PMID: 24001556.
Article19. Bueters T, Ploeger BA, Visser SA. The virtue of translational PKPD modeling in drug discovery: selecting the right clinical candidate while sparing animal lives. Drug Discov Today. 2013; 18(17-18):853–862. PMID: 23665277.
Article20. Fan J, de Lannoy IA. Pharmacokinetics. Biochem Pharmacol. 2014; 87(1):93–120. PMID: 24055064.
Article21. McGonigle P, Ruggeri B. Animal models of human disease: challenges in enabling translation. Biochem Pharmacol. 2014; 87(1):162–171. PMID: 23954708.
Article22. Jiminez JA, Uwiera TC, Douglas Inglis G, Uwiera RR. Animal models to study acute and chronic intestinal inflammation in mammals. Gut Pathog. 2015; 7:29. PMID: 26561503.
Article23. Watanabe K. Prospects for future development in the pharmacology of gastric ulcer models and of gastric acid secretion in experimental animals. Nihon Yakurigaku Zasshi. 1999; 114(3):141–148. PMID: 10553577.24. Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine. 2nd ed. New York: Academic press;2002.25. Lynch CJ. The so-called Swiss mouse. Lab Anim Care. 1969; 19(2):214–220. PMID: 4240230.26. Chia R, Achilli F, Festing MF, Fisher EM. The origins and uses of mouse outbred stocks. Nat Genet. 2005; 37(11):1181–1186. PMID: 16254564.
Article27. Cui S, Chesson C, Hope R. Genetic variation within and between strains of outbred Swiss mice. Lab Anim. 1993; 27(2):116–123. PMID: 8501892.
Article28. Lehoczky JA, Cai WW, Douglas JA, Moran JL, Beier DR, Innis JW. Description and genetic mapping of Polypodia: an X-linked dominant mouse mutant with ectopic caudal limbs and other malformations. Mamm Genome. 2006; 17(9):903–913. PMID: 16964440.
Article29. Jalilzadeh-Amin G, Najarnezhad V, Anassori E, Mostafavi M, Keshipour H. Antiulcer properties of Glycyrrhiza glabra L. extract on experimental models of gastric ulcer in mice. Iran J Pharm Res. 2015; 14(4):1163–1170. PMID: 26664383.30. Martins JL, Rodrigues OR, da Silva DM, Galdino PM, de Paula JR, Romão W, da Costa HB, Vaz BG, Ghedini PC, Costa EA. Mechanisms involved in the gastroprotective activity of Celtis iguanaea (Jacq.) Sargent on gastric lesions in mice. J Ethnopharmacol. 2014; 155(3):1616–1624. PMID: 25153020.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- White Esophageal Mucosa and Black Gastric Mucosa: Upper Gastrointestinal Injury Due to Hydrochloric Acid Ingestion
- Gastroprotective effects of irsogladine maleate on ethanol/hydrochloric acid induced gastric ulcers in mice
- Comparison of the response using ICR mice derived from three different sources to multiple low-dose streptozotocin-induced diabetes mellitus
- Comparison of therapeutic responses to an anticancer drug in three stocks of ICR mice derived from three different sources
- Species Differences in Effect of Ethanol to Urinary Metabolites Excretion of Trichloroethylene in Mice and Rats