Lab Anim Res.
2016 Dec;32(4):194-199. 10.5625/lar.2016.32.4.194.
Ischemic brain injury decreases dynamin-like protein 1 expression in a middle cerebral artery occlusion animal model and glutamate-exposed HT22 cells
- Affiliations
-
- 1Department of Anatomy, College of Veterinary Medicine, Research Institute of Life Science, Gyeongsang National University, Jinju, Korea. pokoh@gnu.ac.kr
- KMID: 2362910
- DOI: http://doi.org/10.5625/lar.2016.32.4.194
Abstract
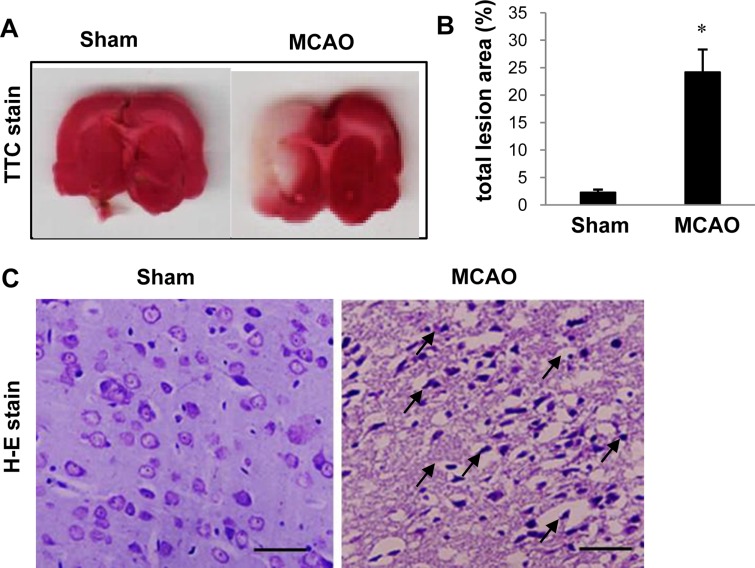
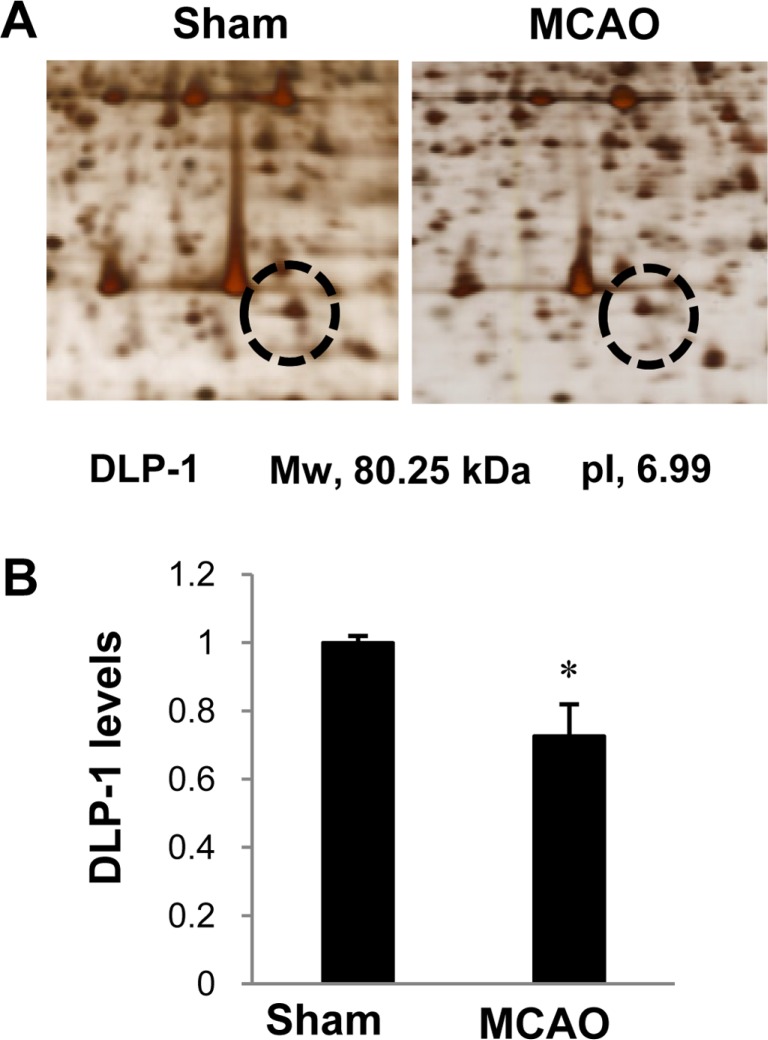
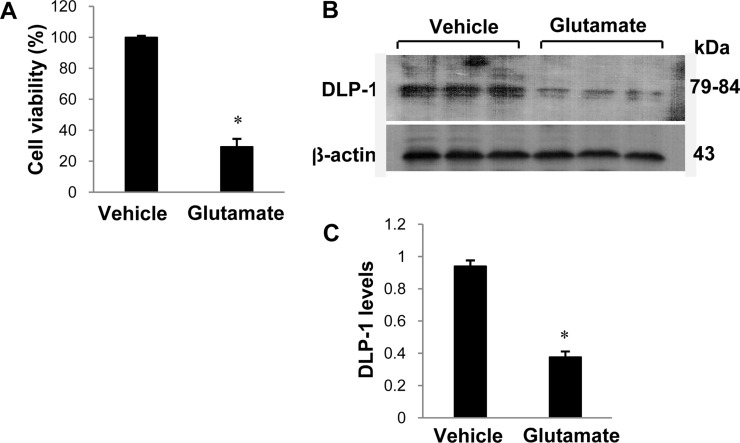
- Dynamin-like protein I (DLP-1) is an important mitochondrial fission and fusion protein that is associated with apoptotic cell death in neurodegenerative diseases. In this study, we investigated DLP-1 expression in a focal cerebral ischemia animal model and glutamate-exposed hippocampal-derived cell line. Middle cerebral artery occlusion (MCAO) was surgically induced in adult male rats to induce focal cerebral ischemic injury. Brain tissues were collected 24 hours after the onset of MCAO. MCAO induces an increase in infarct volume and histopathological changes in the cerebral cortex. We identified a decrease in DLP-1 in the cerebral cortices of MCAO-injured animals using a proteomic approach and Western blot analysis. Moreover, glutamate treatment significantly decreased DLP-1 expression in a hippocampal-derived cell line. The decrease in DLP-1 indicates mitochondrial dysfunction. Thus, these results suggest that neuronal cell injury induces a decrease in DLP-1 levels and consequently leads to neuronal cell death.
MeSH Terms
Figure
Cited by 1 articles
-
Hyperglycemia exacerbates downregulation of dynamin-like protein 1 in ischemic cerebral injury
Dong-Ju Park, Myeong-Ok Kim, Phil-Ok Koh
Lab Anim Res. 2017;33(3):202-208. doi: 10.5625/lar.2017.33.3.202.
Reference
-
1. Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta. 2010; 1802(1):80–91. PMID: 19751827.
Article2. Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004; 36(3-4):257–264. PMID: 15261481.
Article3. Goldenthal MJ, Marín-García J. Mitochondrial signaling pathways: a receiver/integrator organelle. Mol Cell Biochem. 2004; 262(1-2):1–16. PMID: 15532704.
Article4. Abe K, Aoki M, Kawagoe J, Yoshida T, Hattori A, Kogure K, Itoyama Y. Ischemic delayed neuronal death. A mitochondrial hypothesis. Stroke. 1995; 26(8):1478–1489. PMID: 7631357.5. Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005; 47(3):365–378. PMID: 16055061.
Article6. Kageyama Y, Zhang Z, Sesaki H. Mitochondrial division: molecular machinery and physiological functions. Curr Opin Cell Biol. 2011; 23(4):427–434. PMID: 21565481.
Article7. Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao P. Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev. 2011; 67(1-2):103–118. PMID: 21145355.
Article8. Palmer CS, Osellame LD, Stojanovski D, Ryan MT. The regulation of mitochondrial morphology: intricate mechanisms and dynamic machinery. Cell Signal. 2011; 23(10):1534–1545. PMID: 21683788.
Article9. Shirendeb U, Reddy AP, Manczak M, Calkins MJ, Mao P, Tagle DA, Reddy PH. Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington's disease: implications for selective neuronal damage. Hum Mol Genet. 2011; 20(7):1438–1455. PMID: 21257639.
Article10. Swerdlow RH. The neurodegenerative mitochondriopathies. J Alzheimers Dis. 2009; 17(4):737–751. PMID: 19542616.
Article11. Li H, Chen Y, Jones AF, Sanger RH, Collis LP, Flannery R, McNay EC, Yu T, Schwarzenbacher R, Bossy B, Bossy-Wetzel E, Bennett MV, Pypaert M, Hickman JA, Smith PJ, Hardwick JM, Jonas EA. Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2008; 105(6):2169–2174. PMID: 18250306.
Article12. Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009; 11(8):958–966. PMID: 19578372.
Article13. Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, Iijima M, Sesaki H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol. 2009; 186(6):805–816. PMID: 19752021.
Article14. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989; 20(1):84–91. PMID: 2643202.
Article15. Sung JH, Cho EH, Min W, Kim MJ, Kim MO, Jung EJ, Koh PO. Identification of proteins regulated by estradiol in focal cerebral ischemic injury--a proteomics approach. Neurosci Lett. 2010; 477(2):66–71. PMID: 20403413.
Article16. Koh PO. 17Beta-estradiol prevents the glutamate-induced decrease of Akt and its downstream targets in HT22 cells. J Vet Med Sci. 2007; 69(3):285–288. PMID: 17409645.
Article17. Maher P, Davis JB. The role of monoamine metabolism in oxidative glutamate toxicity. J Neurosci. 1996; 16(20):6394–6401. PMID: 8815918.
Article18. Tan S, Sagara Y, Liu Y, Maher P, Schubert D. The regulation of reactive oxygen species production during programmed cell death. J Cell Biol. 1998; 141(6):1423–1432. PMID: 9628898.
Article19. Tiwari BS, Belenghi B, Levine A. Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol. 2002; 128(4):1271–1281. PMID: 11950976.
Article20. Pitts KR, Yoon Y, Krueger EW, McNiven MA. The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol Biol Cell. 1999; 10(12):4403–4417. PMID: 10588666.
Article21. Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM. A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol. 1998; 143(2):351–358. PMID: 9786947.
Article22. Nakahara I, Kikuchi H, Taki W, Nishi S, Kito M, Yonekawa Y, Goto Y, Ogata N. Changes in major phospholipids of mitochondria during postischemic reperfusion in rat brain. J Neurosurg. 1992; 76(2):244–250. PMID: 1309864.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Focal Cerebral Ischemia Induces Decrease of Astrocytic Phosphoprotein PEA-15 in Brain Tissue and HT22 Cells
- Focal cerebral ischemic injury decreases calbindin expression in brain tissue and HT22 cells
- Cerebral ischemic injury decreases α-synuclein expression in brain tissue and glutamate-exposed HT22 cells
- Chlorogenic acid regulates the expression of protein phosphatase 2A subunit B in the cerebral cortex of a rat stroke model and glutamate-exposed neurons
- Focal Cerebral Ischemia Reduces Protein Phosphatase 2A Subunit B Expression in Brain Tissue and HT22 Cells