Lab Anim Res.
2017 Sep;33(3):244-250. 10.5625/lar.2017.33.3.244.
Cerebral ischemic injury decreases α-synuclein expression in brain tissue and glutamate-exposed HT22 cells
- Affiliations
-
- 1Department of Anatomy, College of Veterinary Medicine, Research Institute of Life Science, Gyeongsang National University, Jinju, Korea. pokoh@gnu.ac.kr
- KMID: 2391098
- DOI: http://doi.org/10.5625/lar.2017.33.3.244
Abstract
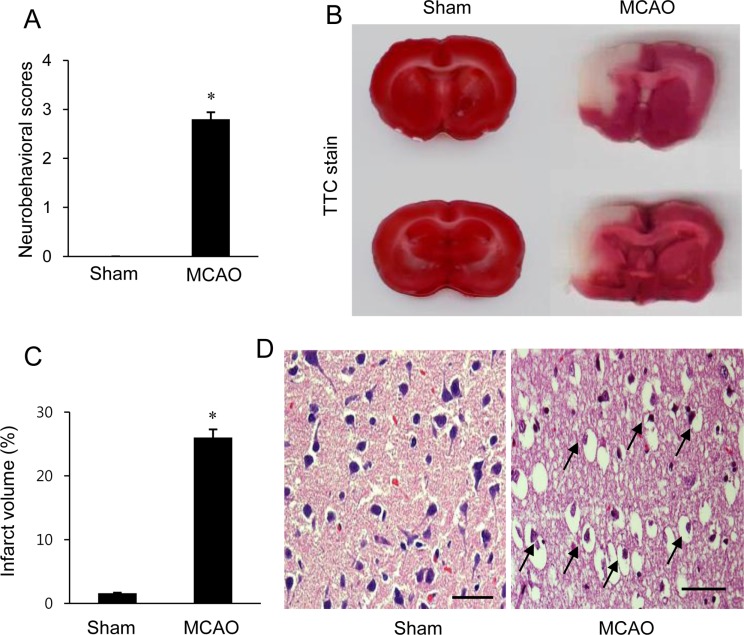
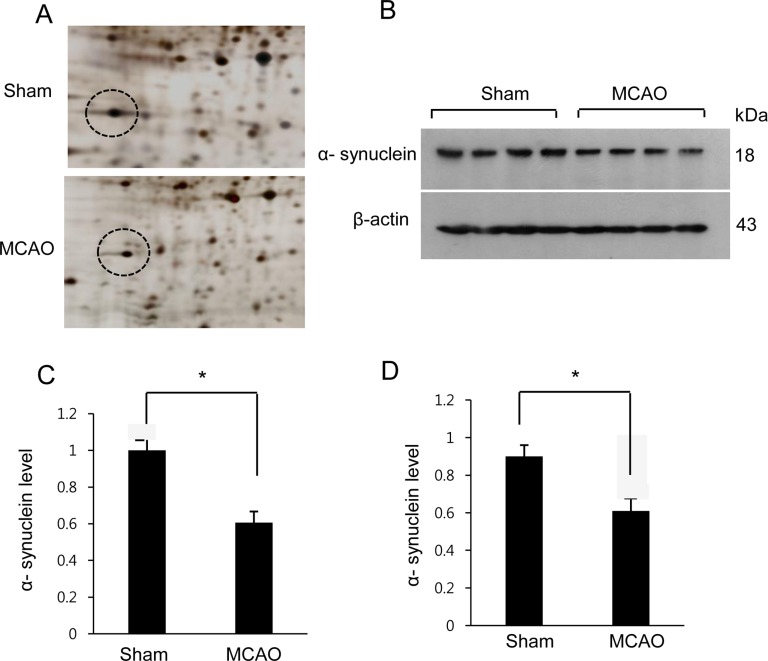
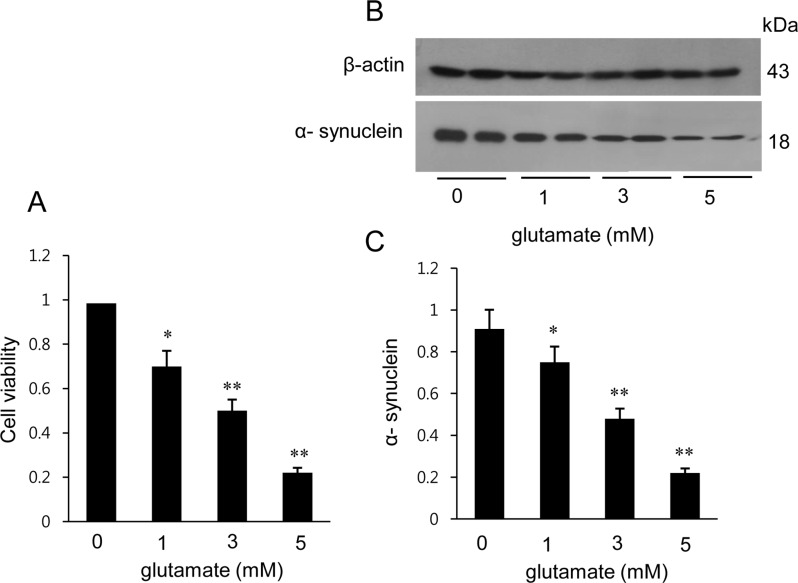
- α-Synuclein is abundantly expressed in neuronal tissue, plays an essential role in the pathogenesis of neurodegenerative disorders, and exerts a neuroprotective effect against oxidative stress. Cerebral ischemia causes severe neurological disorders and neuronal dysfunction. In this study, we examined α-synuclein expression in middle cerebral artery occlusion (MCAO)-induced cerebral ischemic injury and neuronal cells damaged by glutamate treatment. MCAO surgical operation was performed on male Sprague-Dawley rats, and brain samples were isolated 24 hours after MCAO. We confirmed neurological behavior deficit, infarction area, and histopathological changes following MCAO injury. A proteomic approach and Western blot analysis demonstrated a decrease in α-synuclein in the cerebral cortices after MCAO injury. Moreover, glutamate treatment induced neuronal cell death and decreased α-synuclein expression in a hippocampal-derived cell line in a dose-dependent manner. It is known that α-synuclein regulates neuronal survival, and low levels of α-synuclein expression result in cytotoxicity. Thus, these results suggest that cerebral ischemic injury leads to a reduction in α-synuclein and consequently causes serious brain damage.
MeSH Terms
Figure
Cited by 1 articles
-
Hyperglycemia aggravates decrease in alpha-synuclein expression in a middle cerebral artery occlusion model
Ju-Bin Kang, Dong-Kyun Kim, Dong-Ju Park, Murad-Ali Shah, Myeong-Ok Kim, Eun-Jung Jung, Han-Shin Lee, Phil-Ok Koh
Lab Anim Res. 2018;34(4):195-202. doi: 10.5625/lar.2018.34.4.195.
Reference
-
1. Schaller B, Graf R. Cerebral ischemia and reperfusion: the pathophysiologic concept as a basis for clinical therapy. J Cereb Blood Flow Metab. 2004; 24(4):351–371. PMID: 15087705.
Article2. Min D, Mao X, Wu K, Cao Y, Guo F, Zhu S, Xie N, Wang L, Chen T, Shaw C, Cai J. Donepezil attenuates hippocampal neuronal damage and cognitive deficits after global cerebral ischemia in gerbils. Neurosci Lett. 2012; 510(1):29–33. PMID: 22240104.
Article3. Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta. 2010; 1802(1):80–91. PMID: 19751827.
Article4. Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004; 36(3-4):257–264. PMID: 15261481.
Article5. Rink C, Khanna S. MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics. 2011; 43(10):521–528. PMID: 20841499.
Article6. Hayashi T, Abe K. Ischemic neuronal cell death and organellae damage. Neurol Res. 2004; 26(8):827–834. PMID: 15727266.
Article7. Sidhu A, Wersinger C, Vernier P. Does alpha-synuclein modulate dopaminergic synaptic content and tone at the synapse? FASEB J. 2004; 18(6):637–647. PMID: 15054086.8. Zhu M, Qin ZJ, Hu D, Munishkina LA, Fink AL. Alpha-synuclein can function as an antioxidant preventing oxidation of unsaturated lipid in vesicles. Biochemistry. 2006; 45(26):8135–8142. PMID: 16800638.9. Zhu M, Li W, Lu C. Role of alpha-synuclein protein levels in mitochondrial morphology and cell survival in cell lines. PLoS One. 2012; 7(4):e36377. PMID: 22558453.
Article10. Muralikrishna Adibhatla R, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006; 40(3):376–387. PMID: 16443152.11. Adibhatla RM, Hatcher JF. Phospholipase A(2), reactive oxygen species, and lipid peroxidation in CNS pathologies. BMB Rep. 2008; 41(8):560–567. PMID: 18755070.
Article12. Angelova PR, Horrocks MH, Klenerman D, Gandhi S. Abramov AY, Shchepinov MS. Lipid peroxidation is essential for α-synuclein-induced cell death. J Neurochem. 2015; 133(4):582–589. PMID: 25580849.13. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989; 20(1):84–91. PMID: 2643202.
Article14. Maher P, Davis JB. The role of monoamine metabolism in oxidative glutamate toxicity. J Neurosci. 1996; 16(20):6394–6401. PMID: 8815918.
Article15. Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol. 2003; 62(4):329–339. PMID: 12722825.
Article16. Li Y, Chopp M, Powers C, Jiang N. Apoptosis and protein expression after focal cerebral ischemia in rat. Brain Res. 1997; 765(2):301–312. PMID: 9313903.
Article17. Kaplan B, Ratner V, Haas E. Alpha-synuclein: its biological function and role in neurodegenerative diseases. J Mol Neurosci. 2003; 20(2):83–92. PMID: 12794302.18. Ma QL, Chan P, Yoshii M, Uéda K. Alpha-synuclein aggregation and neurodegenerative diseases. J Alzheimers Dis. 2003; 5(2):139–148. PMID: 12719631.19. Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atarés B, Llorens V, Gomez Tortosa E, del Ser T, Muñoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004; 55(2):164–173. PMID: 14755719.20. Lee M, Hyun D, Halliwell B, Jenner P. Effect of the overexpression of wild-type or mutant alpha-synuclein on cell susceptibility to insult. J Neurochem. 2001; 76(4):998–1009. PMID: 11181819.21. Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003; 66(8):1499–1503. PMID: 14555227.
Article22. Seo JH, Rah JC, Choi SH, Shin JK, Min K, Kim HS, Park CH, Kim S, Kim EM, Lee SH, Lee S, Suh SW, Suh YH. Alpha-synuclein regulates neuronal survival via Bcl-2 family expression and PI3/Akt kinase pathway. FASEB J. 2002; 16(13):1826–1828. PMID: 12223445.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Focal Cerebral Ischemia Induces Decrease of Astrocytic Phosphoprotein PEA-15 in Brain Tissue and HT22 Cells
- Focal cerebral ischemic injury decreases calbindin expression in brain tissue and HT22 cells
- Ischemic brain injury decreases dynamin-like protein 1 expression in a middle cerebral artery occlusion animal model and glutamate-exposed HT22 cells
- Chlorogenic acid regulates the expression of protein phosphatase 2A subunit B in the cerebral cortex of a rat stroke model and glutamate-exposed neurons
- Ferulic acid prevents the injury-induced decrease of gamma-enolase expression in brain tissue and HT22 cells