Nutr Res Pract.
2012 Jun;6(3):201-207.
Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice
- Affiliations
-
- 1Department of Smart Foods and Drugs, School of Food and Life Science, Inje University, 607 Obang-dong, Gimhae, Gyungnam 621-749, Korea. fdsnkiji@inje.ac.kr
Abstract
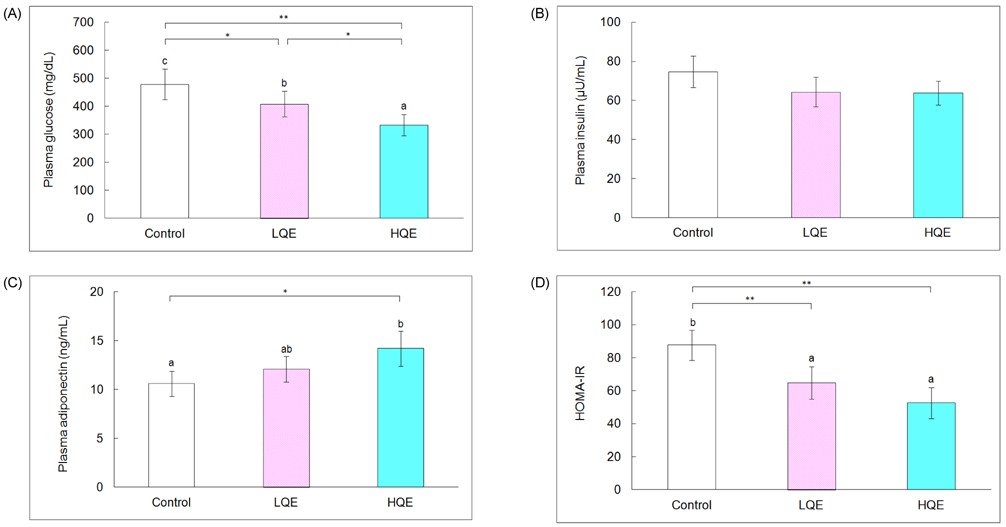
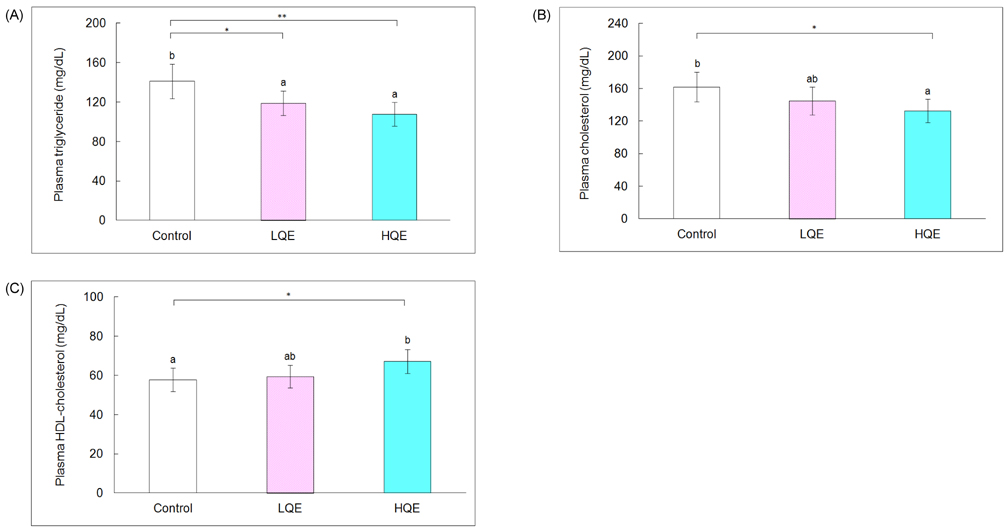
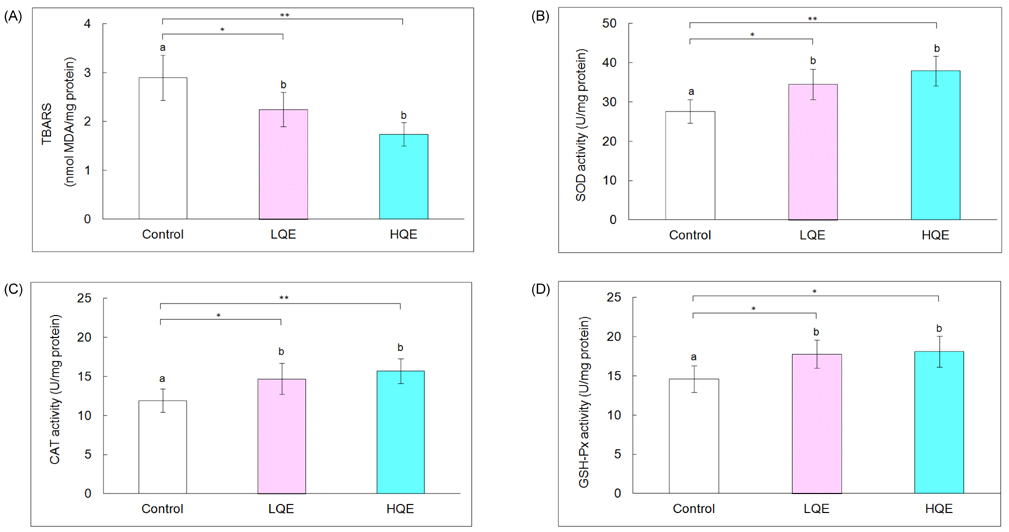
- This study investigated the hypoglycemic, hypolipidemic, and antioxidant effects of dietary quercetin in an animal model of type 2 diabetes mellitus. Four-week-old C57BL/KsJ-db/db mice (n = 18) were offered an AIN-93G diet or a diet containing quercetin at 0.04% (low quercetin, LQE) or 0.08% of the diet (high quercetin, HQE) for 6 weeks after 1 week of adaptation. Plasma glucose, insulin, adiponectin, and lipid profiles, and lipid peroxidation of the liver were determined. Plasma glucose levels were significantly lower in the LQE group than in the control group, and those in the HQE group were even further reduced compared with the LQE group. The homeostasis model assessment for insulin resistance (HOMA-IR) showed lower values for LQE and HQE than for the control group without significant influence on insulin levels. High quercetin increased plasma adiponectin compared with the control group. Plasma triglycerides in the LQE and HQE groups were lower than those in the control group. Supplementation with high quercetin decreased plasma total cholesterol and increased HDL-cholesterol compared with the control group. Consumption of low and high quercetin reduced thiobarbituric acid reactive substances (TBARS) levels and elevated activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) in the liver. Thus, quercetin could be effective in improving hyperglycemia, dyslipidemia, and antioxidant status in type 2 diabetes.
Keyword
MeSH Terms
-
Adiponectin
Animals
Antioxidants
Catalase
Cholesterol
Diabetes Mellitus, Type 2
Diet
Dyslipidemias
Glucose
Glutathione Peroxidase
Homeostasis
Hyperglycemia
Insulin
Insulin Resistance
Lipid Peroxidation
Liver
Mice
Models, Animal
Plasma
Quercetin
Superoxide Dismutase
Thiobarbiturates
Thiobarbituric Acid Reactive Substances
Triglycerides
Adiponectin
Antioxidants
Catalase
Cholesterol
Glucose
Glutathione Peroxidase
Insulin
Quercetin
Superoxide Dismutase
Thiobarbiturates
Thiobarbituric Acid Reactive Substances
Triglycerides
Figure
Reference
-
1. Cheng D. Prevalence, predisposition and prevention of type II diabetes. Nutr Metab (Lond). 2005. 2:29.
Article2. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998. 21:1414–1431.
Article3. O'Keefe JH Jr, Miles JM, Harris WH, Moe RM, McCallister BD. Improving the adverse cardiovascular prognosis of type 2 diabetes. Mayo Clin Proc. 1999. 74:171–180.4. Maritim AC, Sanders RA, Watkins JB 3rd. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003. 17:24–38.
Article5. Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005. 59:365–373.
Article6. Garg A, Grundy SM. Management of dyslipidemia in NIDDM. Diabetes Care. 1990. 13:153–169.
Article7. American Diabetes Association. Management of dyslipidemia in adults with diabetes. Diabetes Care. 2003. 26:Suppl 1. 583–586.8. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010. 107:1058–1070.
Article9. Rao YK, Geethangili M, Fang SH, Tzeng YM. Antioxidant and cytotoxic activities of naturally occurring phenolic and related compounds: a comparative study. Food Chem Toxicol. 2007. 45:1770–1776.
Article10. Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008. 585:325–337.
Article11. Mahesh T, Menon VP. Quercetin allievates oxidative stress in streptozotocin-induced diabetic rats. Phytother Res. 2004. 18:123–127.
Article12. Dias AS, Porawski M, Alonso M, Marroni N, Collado PS, González-Gallego J. Quercetin decreases oxidative stress, NF-kappaB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J Nutr. 2005. 135:2299–2304.
Article13. Kobori M, Masumoto S, Akimoto Y, Oike H. Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a Western-style diet in C57/BL6J mice. Mol Nutr Food Res. 2011. 55:530–540.
Article14. Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003. 135C:357–364.
Article15. Torres-Piedra M, Ortiz-Andrade R, Villalobos-Molina R, Singh N, Medina-Franco JL, Webster SP, Binnie M, Navarrete-Vázquez G, Estrada-Soto S. A comparative study of flavonoid analogues on streptozotocin-nicotinamide induced diabetic rats: quercetin as a potential antidiabetic agent acting via 11beta-hydroxysteroid dehydrogenase type 1 inhibition. Eur J Med Chem. 2010. 45:2606–2612.
Article16. Ishikawa A, Yamashita H, Hiemori M, Inagaki E, Kimoto M, Okamoto M, Tsuji H, Memon AN, Mohammadio A, Natori Y. Characterization of inhibitors of postprandial hyperglycemia from the leaves of Nerium indicum. J Nutr Sci Vitaminol (Tokyo). 2007. 53:166–173.
Article17. Jo SH, Ka EH, Lee HS, Apostolidis E, Jang HD, Kwon YI. Comparison of antioxidant potential and rat intestinal α-glucosidases inhibitory activities of quercetin, rutin, and isoquercetin. Int J Applied Res Nat Prod. 2009. 2:52–60.18. Kim JH, Kang MJ, Choi HN, Jeong SM, Lee YM, Kim JI. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr Res Pract. 2011. 5:107–111.
Article19. Ramachandra R, Shetty AK, Salimath PV. Quercetin alleviates activities of intestinal and renal disaccharidases in streptozotocin-induced diabetic rats. Mol Nutr Food Res. 2005. 49:355–360.
Article20. Shetty AK, Rashmi R, Rajan MGR, Sambaiah K, Salimath PV. Antidiabetic influence of quercetin in streptozotocin-induced diabetic rats. Nutr Res. 2004. 24:373–381.
Article21. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993. 123:1939–1951.
Article22. Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care. 1997. 20:1087–1092.
Article23. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979. 95:351–358.
Article24. Aebi H. Bergmeyer HU, editor. Catalase. Methods of Enzymatic Analysis. 1974. New York: Academic Press;673–683.
Article25. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967. 70:158–169.26. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974. 47:469–474.
Article27. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976. 72:248–254.
Article28. Standl E, Baumgartl HJ, Füchtenbusch M, Stemplinger J. Effect of acarbose on additional insulin therapy in type 2 diabetic patients with late failure of sulphonylurea therapy. Diabetes Obes Metab. 1999. 1:215–220.
Article29. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008. 294:E15–E26.
Article30. Sheng T, Yang K. Adiponectin and its association with insulin resistance and type 2 diabetes. J Genet Genomics. 2008. 35:321–326.
Article31. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993. 329:977–986.32. Gnoni GV, Paglialonga G, Siculella L. Quercetin inhibits fatty acid and triacylglycerol synthesis in rat-liver cells. Eur J Clin Invest. 2009. 39:761–768.
Article33. Azuma K, Toyofuku Y, Iesaki T, Otsuka A, Tanaka A, Mita T, Hirose T, Tanaka Y, Daida H, Kawamori R, Watada H. Acarbose, an alpha-glucosidase inhibitor, improves endothelial dysfunction in Goto-Kakizaki rats exhibiting repetitive blood glucose fluctuation. Biochem Biophys Res Commun. 2006. 345:688–693.
Article34. Haffner SM. Management of dyslipidemia in adults with diabetes. Diabetes Care. 1998. 21:160–178.
Article35. Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993. 16:434–444.
Article36. Witztum JL, Steinberg D. The oxidative modification hypothesis of atherosclerosis: does it hold for humans? Trends Cardiovasc Med. 2001. 11:93–102.
Article37. Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest. 2006. 116:1071–1080.
Article38. Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic Biol Med. 1994. 16:845–850.
Article39. Galati G, O'Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med. 2004. 37:287–303.
Article40. Harman D. The aging process: major risk factor for disease and death. Proc Natl Acad Sci U S A. 1991. 88:5360–5363.
Article41. Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002. 82:47–95.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sargassum coreanum extract alleviates hyperglycemia and improves insulin resistance in db/db diabetic mice
- Daraesoon (shoot of hardy kiwi) mitigates hyperglycemia in db/db mice by alleviating insulin resistance and inflammation
- Effect of glucagon-like peptide 1 on salivary gland hypofunction in diabetic db/db mice
- Coating rice with mulberry leaves rich in deoxynojirimycin ameliorates hyperglycemia and dyslipidemia in C57BL/KsJ db/db mice
- Gynura procumbens extract improves insulin sensitivity and suppresses hepatic gluconeogenesis in C57BL/KsJ-db/db mice