Nutr Res Pract.
2016 Oct;10(5):507-515. 10.4162/nrp.2016.10.5.507.
Gynura procumbens extract improves insulin sensitivity and suppresses hepatic gluconeogenesis in C57BL/KsJ-db/db mice
- Affiliations
-
- 1Department of Food Science and Nutrition, Pusan National University, Jangjeon 2-dong, Geumjeong-gu, Busan 46241, Korea. hanjs@pusan.ac.kr
- 2Research Institute of Ecology for the Elderly, Pusan National University, Busan 46241, Korea.
- KMID: 2434085
- DOI: http://doi.org/10.4162/nrp.2016.10.5.507
Abstract
- BACKGROUND/OBJECTIVES
This study was designed to investigate whether Gynura procumbens extract (GPE) can improve insulin sensitivity and suppress hepatic glucose production in an animal model of type 2 diabetes.
MATERIALS/METHODS
C57BL/Ksj-db/db mice were divided into 3 groups, a regular diet (control), GPE, and rosiglitazone groups (0.005 g/100 g diet) and fed for 6 weeks.
RESULTS
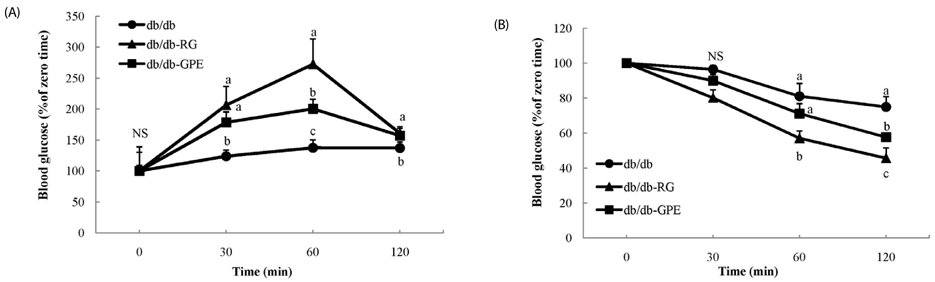
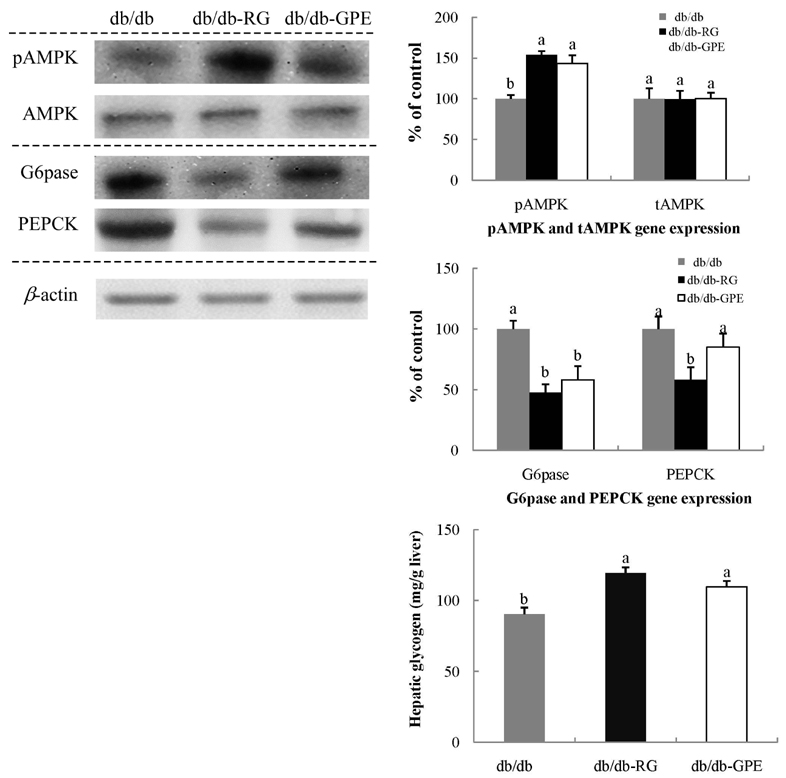
Mice supplemented with GPE showed significantly lower blood levels of glucose and glycosylated hemoglobin than diabetic control mice. Glucose and insulin tolerance test also showed the positive effect of GPE on increasing insulin sensitivity. The homeostatic index of insulin resistance was significantly lower in mice supplemented with GPE than in the diabetic control mice. In the skeletal muscle, the expression of phosphorylated AMP-activated protein kinase, pAkt substrate of 160 kDa, and PM-glucose transporter type 4 increased in mice supplemented with GPE when compared to that of the diabetic control mice. GPE also decreased the expression of glucose-6-phosphatase and phosphoenolpyruvate carboxykinase in the liver.
CONCLUSIONS
These findings demonstrate that GPE might improve insulin sensitivity and inhibit gluconeogenesis in the liver.
MeSH Terms
Figure
Cited by 1 articles
-
Retraction: Gynura procumbens extract improves insulin sensitivity and suppresses hepatic gluconeogenesis in C57BL/KsJ-db/db mice
Sung-In Choi, Hyun-Ah Lee, Ji-Sook Han
Nutr Res Pract. 2019;13(1):76-76. doi: 10.4162/nrp.2019.13.1.76.
Reference
-
1. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010; 87:4–14.
Article2. Bell DS, O'Keefe JH, Jellinger P. Postprandial dysmetabolism: the missing link between diabetes and cardiovascular events? Endocr Pract. 2008; 14:112–124.
Article3. Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes. 1999; 48:937–942.
Article4. Zheng J, Woo SL, Hu X, Botchlett R, Chen L, Huo Y, Wu C. Metformin and metabolic diseases: a focus on hepatic aspects. Front Med. 2015; 9:173–186.
Article5. Derosa G, Maffioli P. α-Glucosidase inhibitors and their use in clinical practice. Arch Med Sci. 2012; 8:899–906.6. Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA. 2002; 287:360–372.7. Baus D, Heermeier K, De Hoop M, Metz-Weidmann C, Gassenhuber J, Dittrich W, Welte S, Tennagels N. Identification of a novel AS160 splice variant that regulates GLUT4 translocation and glucose-uptake in rat muscle cells. Cell Signal. 2008; 20:2237–2246.
Article8. Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jørgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes. 2006; 55:2051–2058.
Article9. Hajiaghaalipour F, Khalilpourfarshbafi M, Arya A. Modulation of glucose transporter protein by dietary flavonoids in type 2 diabetes mellitus. Int J Biol Sci. 2015; 11:508–524.
Article10. Jung UJ, Lee MK, Jeong KS, Choi MS. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J Nutr. 2004; 134:2499–2503.
Article11. Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001; 414:799–806.
Article12. Perry LM, Metzger J. Medicinal Plants of East and Southeast Asia: Attributed Properties and Uses. Cambridge (MA): MIT Press;1980.13. Kim MJ, Lee HJ, Wiryowidagdo S, Kim HK. Antihypertensive effects of Gynura procumbens extract in spontaneously hypertensive rats. J Med Food. 2006; 9:587–590.
Article14. Akowuah GA, Mariam A, Chin JH. The effect of extraction temperature on total phenols and antioxidant activity of Gynura procumbens leaf. Pharmacogn Mag. 2009; 17:81–85.15. Iskander MN, Song Y, Coupar IM, Jiratchariyakul W. Antiinflammatory screening of the medicinal plant Gynura procumbens. Plant Foods Hum Nutr. 2002; 57:233–244.
Article16. Kaewseejan N, Puangpronpitag D, Nakornriab M. Evaluation of phytochemical composition and antibacterial property of Gynura procumbens extract. Asian J Plant Sci. 2012; 11:77–82.
Article17. Lee HW, Hakim P, Rabu A, Sani HA. Antidiabetic effect of Gynura procumbens leaves extracts involve modulation of hepatic carbohydrate metabolism in streptozotocin-induced diabetic rats. J Med Plants Res. 2012; 6:796–812.18. Algariri K, Meng KY, Atangwho IJ, Asmawi MZ, Sadikun A, Murugaiyah V, Ismail N. Hypoglycemic and anti-hyperglycemic study of Gynura procumbens leaf extracts. Asian Pac J Trop Biomed. 2013; 3:358–366.
Article19. Kaewseejan N, Sutthikhum V, Siriamornpun S. Potential of Gynura procumbens leaves as source of flavonoid-enriched fractions with enhanced antioxidant capacity. J Funct Foods. 2015; 12:120–128.
Article20. Seifter S, Dayton S. The estimation of glycogen with the anthrone reagent. Arch Biochem. 1950; 25:191–200.21. Baron AD, Zhu JS, Zhu JH, Weldon H, Maianu L, Garvey WT. Glucosamine induces insulin rsistance in vivo by affecting GLUT 4 translocation in skeletal muscle. J Clin Invest. 1995; 96:2792–2801.
Article22. Klip A, Ramlal T, Young DA, Holloszy JO. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 1987; 224:224–230.
Article23. Klip A, Pâquet MR. Glucose transport and glucose transporters in muscle and their metabolic regulation. Diabetes Care. 1990; 13:228–243.
Article24. Goyal A, Singh S, Tandon N, Gupta N, Gupta YK. Effect of atorvastatin on pancreatic Beta-cell function and insulin resistance in type 2 diabetes mellitus patients: a randomized pilot study. Can J Diabetes. 2014; 38:466–472.
Article25. Fonseca V. Effect of thiazolidinediones on body weight in patients with diabetes mellitus. Am J Med. 2003; 115:Suppl 8A. 42S–48S.
Article26. Fujiwara T, Yoshioka S, Yoshioka T, Ushiyama I, Horikoshi H. Characterization of new oral antidiabetic agent CS-045. Studies in KK and ob/ob mice and Zucker fatty rats. Diabetes. 1988; 37:1549–1558.
Article27. Chou W, Chung MH, Wang HY, Chen JH, Chen WL, Guo HR, Lin HJ, Su SB, Huang CC, Hsu CC. Clinical characteristics of hyperglycemic crises in patients without a history of diabetes. J Diabetes Investig. 2014; 5:657–662.
Article28. Zephy D, Ahmad J. Type 2 diabetes mellitus: role of melatonin and oxidative stress. Diabetes Metab Syndr. 2015; 9:127–131.
Article29. Goldstein DE, Peth SB, England JD, Hess RL, Da Costa J. Effects of acute changes in blood glucose on HbA1c. Diabetes. 1980; 29:623–628.
Article30. Twomey PJ, Viljoen A, Reynolds TM, Wierzbicki AS. Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care. 2004; 27:2569–2570.
Article31. Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003; 135C:357–364.
Article32. Alkhalidy H, Moore W, Zhang Y, McMillan R, Wang A, Ali M, Suh KS, Zhen W, Cheng Z, Jia Z, Hulver M, Liu D. Small molecule kaempferol promotes insulin sensitivity and preserved pancreatic β-cell mass in middle-aged obese diabetic mice. J Diabetes Res. 2015; 2015:532984.33. Orland MJ, Permutt MA. Quantitative analysis of pancreatic proinsulin mRNA in genetically diabetic (db/db) mice. Diabetes. 1987; 36:341–347.
Article34. Kannel WB, Wilson PW, Zhang TJ. The epidemiology of impaired glucose tolerance and hypertension. Am Heart J. 1991; 121:1268–1273.
Article35. Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio heart study. Diabetes Care. 1997; 20:1087–1092.
Article36. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000; 85:2402–2410.
Article37. Dohm GL. Invited review: Regulation of skeletal muscle GLUT-4 expression by exercise. J Appl Physiol (1985). 2002; 93:782–787.
Article38. Ojuka EO, Goyaram V, Smith JA. The role of CaMKII in regulating GLUT4 expression in skeletal muscle. Am J Physiol Endocrinol Metab. 2012; 303:E322–E331.
Article39. Hardie DG. Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 2008; 582:81–89.
Article40. Larance M, Ramm G, Stöckli J, van Dam EM, Winata S, Wasinger V, Simpson F, Graham M, Junutula JR, Guilhaus M, James DE. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J Biol Chem. 2005; 280:37803–37813.
Article41. Eid HM, Martineau LC, Saleem A, Muhammad A, Vallerand D, Benhaddou-Andaloussi A, Nistor L, Afshar A, Arnason JT, Haddad PS. Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitis-idaea. Mol Nutr Food Res. 2010; 54:991–1003.
Article42. Wu C, Zhang X, Zhang X, Luan H, Sun G, Sun X, Wang X, Guo P, Xu X. The caffeoylquinic acid-rich Pandanus tectorius fruit extract increases insulin sensitivity and regulates hepatic glucose and lipid metabolism in diabetic db/db mice. J Nutr Biochem. 2014; 25:412–419.
Article43. Viollet B, Foretz M, Guigas B, Horman S, Dentin R, Bertrand L, Hue L, Andreelli F. Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J Physiol. 2006; 574:41–53.
Article44. Takikawa M, Inoue S, Horio F, Tsuda T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J Nutr. 2010; 140:527–533.
Article45. Hijmans BS, Grefhorst A, Oosterveer MH, Groen AK. Zonation of glucose and fatty acid metabolism in the liver: mechanism and metabolic consequences. Biochimie. 2014; 96:121–129.
Article46. Baik SH. The genes of hepatic glucose metabolism and insulin signaling. J Korean Diabetes Assoc. 1999; 23:1–6.47. Ahn J, Um MY, Lee H, Jung CH, Heo SH, Ha TY. Eleutheroside E, an active component of Eleutherococcus senticosus, ameliorates insulin resistance in type 2 diabetic db/db mice. Evid Based Complement Alternat Med. 2013; 2013:934183.48. Yarushkin AA, Kachaylo EM, Pustylnyak VO. The constitutive androstane receptor activator 4-[(4R,6R)-4,6-diphenyl-1,3-dioxan-2-yl]-N,N-dimethylaniline inhibits the gluconeogenic genes PEPCK and G6Pase through the suppression of HNF4alpha and FOXO1 transcriptional activity. Br J Pharmacol. 2013; 168:1923–1932.
Article49. Eid HM, Nachar A, Thong F, Sweeney G, Haddad PS. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn Mag. 2015; 11:74–81.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retraction: Gynura procumbens extract improves insulin sensitivity and suppresses hepatic gluconeogenesis in C57BL/KsJ-db/db mice
- Sargassum coreanum extract alleviates hyperglycemia and improves insulin resistance in db/db diabetic mice
- Anti-hyperglycemic effects and signaling mechanism of Perilla frutescens sprout extract
- Betaine Alleviates Hypertriglycemia and Tau Hyperphosphorylation in db/db Mice
- Daraesoon (shoot of hardy kiwi) mitigates hyperglycemia in db/db mice by alleviating insulin resistance and inflammation