Korean Circ J.
2014 Mar;44(2):97-104. 10.4070/kcj.2014.44.2.97.
Apoptosis and Inflammation Associated Gene Expressions in Monocrotaline-Induced Pulmonary Hypertensive Rats after Bosentan Treatment
- Affiliations
-
- 1Department of Pediatrics, Ewha Womans University School of Medicine, Seoul, Korea.
- 2Department of Physiology, Ewha Womans University School of Medicine, Seoul, Korea.
- 3Department of Thoracic & Cardiovascular Surgery, Ewha Womans University School of Medicine, Seoul, Korea. mdkkchang@ewha.ac.kr
- 4Ewha Womans University Global Top 5 Research Program, Ewha Womans University School of Medicine, Seoul, Korea.
- KMID: 2223898
- DOI: http://doi.org/10.4070/kcj.2014.44.2.97
Abstract
- BACKGROUND AND OBJECTIVES
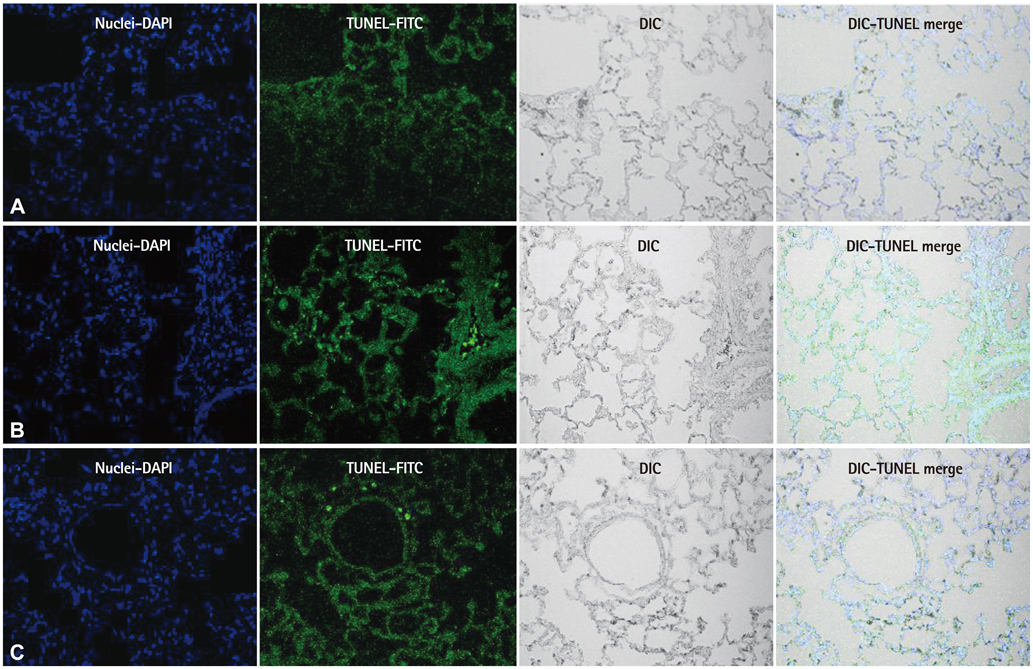
Vascular wall remodeling in pulmonary hypertension can be caused by an aberration in the normal balance between proliferation and apoptosis of endothelial cell in the pulmonary artery. The objective of this study was to evaluate the effect of bosentan on apoptosis in monocrotaline (MCT)-induced pulmonary hypertension.
MATERIALS AND METHODS
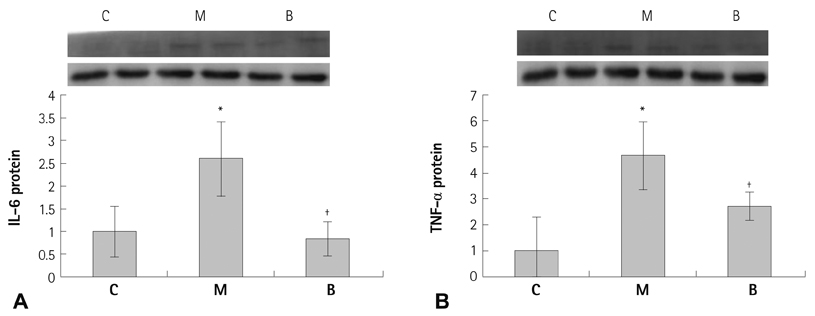
Sprague-Dawley rats were divided into three groups: control (C) group, M group (MCT 60 mg/kg) and B group (MCT 60 mg/kg plus bosentan 20 mg/day orally). Gene expressions of Bcl (B cell leukemia/lymphoma)-2, caspase-3, complement component (C)-6, vascular endothelial growth factor (VEGF), interleukin (IL)-6 and tumor necrosis factor-alpha (TNF-alpha) were analyzed by real time polymerase chain reaction and western blot analysis.
RESULTS
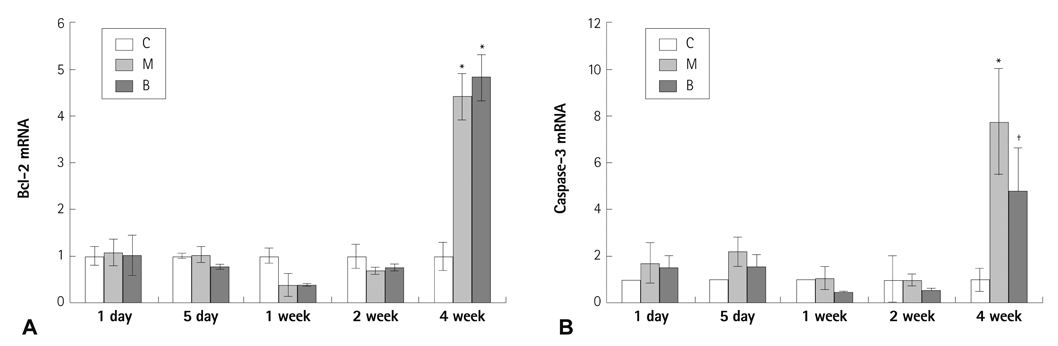
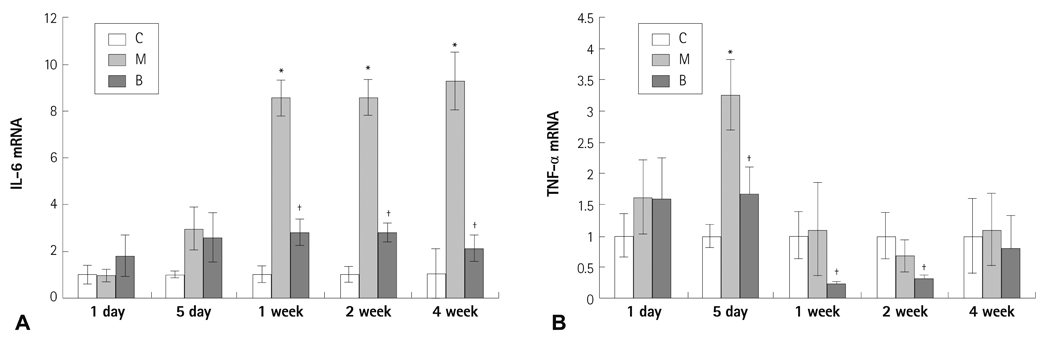
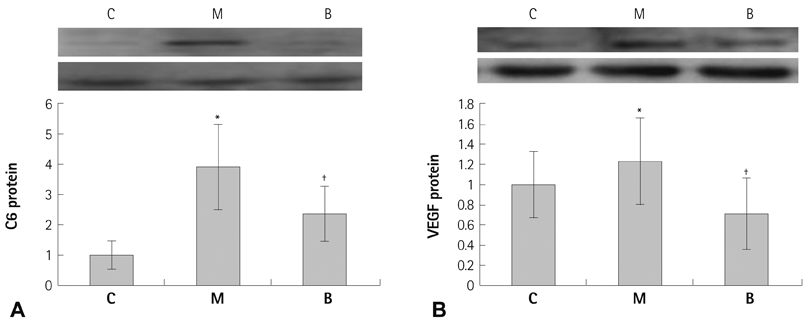
The messenger ribonucleic acid (mRNA) expressions of caspase-3 and VEGF were significantly increased in the M group compared with the C group, and significantly decreased in the B group compared with the M group in week 4. mRNA expression of IL-6 was significantly decreased in weeks 1, 2, and 4 in the B group compared with the M group. mRNA expression of TNF-alpha was significantly decreased on day 5 and in weeks 1 and 2 in the B group compared with the M group.
CONCLUSION
Bosentan may have potential for preventing apoptosis and inflammation.
MeSH Terms
-
Animals
Apoptosis*
Blotting, Western
Caspase 3
Complement System Proteins
Endothelial Cells
Gene Expression*
Hypertension, Pulmonary
Inflammation*
Interleukin-6
Interleukins
Monocrotaline
Pulmonary Artery
Rats*
Rats, Sprague-Dawley
Real-Time Polymerase Chain Reaction
RNA
RNA, Messenger
Tumor Necrosis Factor-alpha
Vascular Endothelial Growth Factor A
Caspase 3
Complement System Proteins
Interleukin-6
Interleukins
Monocrotaline
RNA
RNA, Messenger
Tumor Necrosis Factor-alpha
Vascular Endothelial Growth Factor A
Figure
Cited by 1 articles
-
Protective Effect of Right Ventricular Mitochondrial Damage by Cyclosporine A in Monocrotaline-induced Pulmonary Hypertension
Dong Seok Lee, Yong Wook Jung
Korean Circ J. 2018;48(12):1135-1144. doi: 10.4070/kcj.2018.0061.
Reference
-
1. Gaine S. Pulmonary hypertension. JAMA. 2000; 284:3160–3168.2. Jurasz P, Courtman D, Babaie S, Stewart DJ. Role of apoptosis in pulmonary hypertension: from experimental models to clinical trials. Pharmacol Ther. 2010; 126:1–8.3. Denecker G, Vercammen D, Declercq W, Vandenabeele P. Apoptotic and necrotic cell death induced by death domain receptors. Cell Mol Life Sci. 2001; 58:356–370.4. Kuwano K, Hara N. Signal transduction pathways of apoptosis and inflammation induced by the tumor necrosis factor receptor family. Am J Respir Cell Mol Biol. 2000; 22:147–149.5. Sage E, Mercier O, Van den Eyden F, et al. Endothelial cell apoptosis in chronically obstructed and reperfused pulmonary artery. Respir Res. 2008; 9:19.6. Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004; 109:159–165.7. Lévy M, Maurey C, Celermajer DS, et al. Impaired apoptosis of pulmonary endothelial cells is associated with intimal proliferation and irreversibility of pulmonary hypertension in congenital heart disease. J Am Coll Cardiol. 2007; 49:803–810.8. Takahashi H, Goto N, Kojima Y, et al. Downregulation of type II bone morphogenetic protein receptor in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006; 290:L450–L458.9. Jamieson SW, Kapelanski DP, Sakakibara N, et al. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg. 2003; 76:1457–1462.10. Hilliker KS, Roth RA. Increased vascular responsiveness in lungs of rats with pulmonary hypertension induced by monocrotaline pyrrole. Am Rev Respir Dis. 1985; 131:46–50.11. Kido M, Hirose T, Tanaka K, Kurozumi T, Shoyama Y. Increased alveolarcapillary membrane permeability by monocrotaline. Jpn J Med. 1981; 20:170–179.12. Sugita T, Stenmark KR, Wagner WW Jr, et al. Abnormal alveolar cells in monocrotaline induced pulmonary hypertension. Exp Lung Res. 1983; 5:201–215.13. Rosenberg HC, Rabinovitch M. Endothelial injury and vascular reactivity in monocrotaline pulmonary hypertension. Am J Physiol. 1988; 255:H1484–H1491.14. Wilson DW, Lamé MW, Dunston SK, Segall HJ. DNA damage cell checkpoint activities are altered in monocrotaline pyrrole-induced cell cycle arrest in human pulmonary artery endothelial cells. Toxicol Appl Pharmacol. 2000; 166:69–80.15. Lim KA, Kim KC, Cho MS, Lee BE, Kim HS, Hong YM. Gene expression of endothelin-1 and endothelin receptor a on monocrotaline-induced pulmonary hypertension in rats after bosentan treatment. Korean Circ J. 2010; 40:459–464.16. Koo HS, Kim KC, Hong YM. Gene expressions of nitric oxide synthase and matrix metalloproteinase-2 in monocrotaline-induced pulmonary hypertension in rats after bosentan treatment. Korean Circ J. 2011; 41:83–90.17. Stier S, Totzke G, Gruewald E, et al. Identification of p54nrb and the 14-3-3 Protein HS1 as TNF-alpha-inducible genes related to cell cycle control and apoptosis in human arterial endothelial cells. J Biochem Mol Biol. 2005; 38:447–456.18. Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J. 2005; 19:1178–1180.19. Matter CM, Chadjichristos CE, Meier P, et al. Role of endogenous Fas (CD95/Apo-1) ligand in balloon-induced apoptosis, inflammation, and neointima formation. Circulation. 2006; 113:1879–1887.20. Partovian C, Adnot S, Eddahibi S, et al. Heart and lung VEGF mRNA expression in rats with monocrotaline- or hypoxia-induced pulmonary hypertension. Am J Physiol. 1998; 275:H1948–H1956.21. Alavi A, Hood JD, Frausto R, Stupack DG, Cheresh DA. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 2003; 301:94–96.22. VEGF Inhibition Study in Ocular Neovascularization (V.I.S.I.O.N.) Clinical Trial Group. D'Amico DJ, Masonson HN, et al. Pegaptanib sodium for neovascular age-related macular degeneration: two-year safety results of the two prospective, multicenter, controlled clinical trials. Ophthalmology. 2006; 113:992–1001.23. Jankov RP, Kantores C, Belcastro R, Yi M, Tanswell AK. Endothelin-1 inhibits apoptosis of pulmonary arterial smooth muscle in the neonatal rat. Pediatr Res. 2006; 60:245–251.24. Hahn AW, Resink TJ, Scott-Burden T, Powell J, Dohi Y, Bühler FR. Stimulation of endothelin mRNA and secretion in rat vascular smooth muscle cells: a novel autocrine function. Cell Regul. 1990; 1:649–659.25. Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993; 328:1732–1739.26. Jasmin JF, Lucas M, Cernacek P, Dupuis J. Effectiveness of a nonselective ET(A/B) and a selective ET(A) antagonist in rats with monocrotaline-induced pulmonary hypertension. Circulation. 2001; 103:314–318.27. Jankov RP, Luo X, Belcastro R, et al. Gadolinium chloride inhibits pulmonary macrophage influx and prevents O2-induced pulmonary hypertension in the neonatal rat. Pediatr Res. 2001; 50:172–183.28. Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2008; 118:2372–2379.29. Soon E, Holmes AM, Treacy CM, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010; 122:920–927.30. Price LC, Wort SJ, Perros F, et al. Inflammation in pulmonary arterial hypertension. Chest. 2012; 141:210–221.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Bosentan Treatment on Angiotensin Converting Enzyme in Monocrotaline Induced Pulmonary Hypertension Rats
- Gene Expression of Endothelin-1 and Endothelin Receptor A on Monocrotaline-Induced Pulmonary Hypertension in Rats After Bosentan Treatment
- Gene Expressions of Nitric Oxide Synthase and Matrix Metalloproteinase-2 in Monocrotaline-Induced Pulmonary Hypertension in Rats After Bosentan Treatment
- Effect of endothelin receptor blockade on monocrotaline-induced pulmonary hypertension in rats
- Angiotensin-(1-9) ameliorates pulmonary arterial hypertension via angiotensin type II receptor