Gene Expression of Endothelin-1 and Endothelin Receptor A on Monocrotaline-Induced Pulmonary Hypertension in Rats After Bosentan Treatment
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, CHA University, Pocheon, Korea.
- 2Department of Thoracic and Cardiovascular Surgery, School of Medicine, Ewha Womans University, Seoul, Korea.
- 3Department of Pathology, School of Medicine, Ewha Womans University, Seoul, Korea.
- 4Department of Preventive Medicine, School of Medicine, Ewha Womans University, Seoul, Korea.
- 5Department of Pediatrics, School of Medicine, Ewha Womans University, Seoul, Korea. ymhong@ewha.ac.kr
- KMID: 1456139
- DOI: http://doi.org/10.4070/kcj.2010.40.9.459
Abstract
- BACKGROUND AND OBJECTIVES
Endothelin (ET)-1, a potent endothelium-derived vasoconstrictor peptide, has a potential pathophysiologic role in pulmonary hypertension. Bosentan, a dual ET receptor (ET(A)/ET(B)) antagonist, is efficacious in treatment of pulmonary hypertension. The objectives of this study were to investigate the expression of ET-1 and ET receptor A (ERA) genes and to evaluate the effect of bosentan in monocrotaline (MCT)-induced pulmonary hypertension.
MATERIALS AND METHODS
Four-week-old male Sprague-Dawley rats were treated as follows: control (n=36), subcutaneous (sc) injection of saline; MCT (n=36), sc injection of MCT (60 mg/kg); and bosentan (n=36), sc injection of MCT (60 mg/kg) plus 25 mg/kg/day bosentan orally.
RESULTS
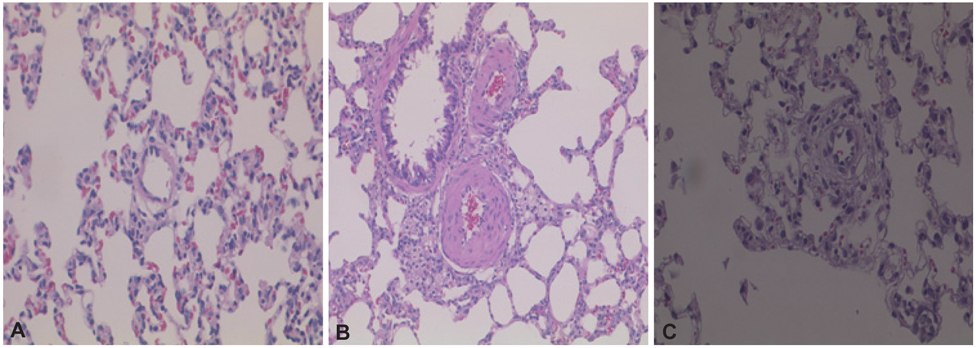
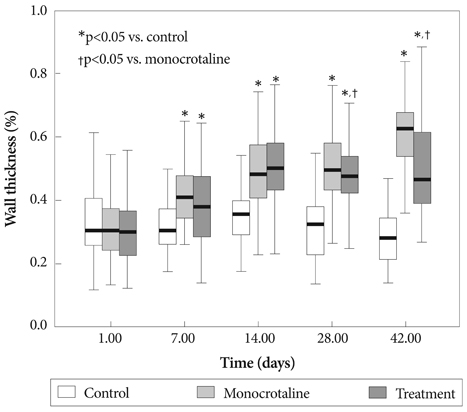
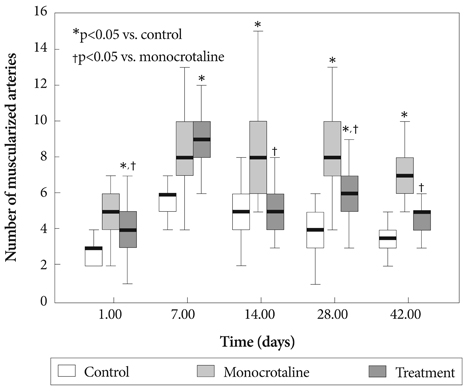
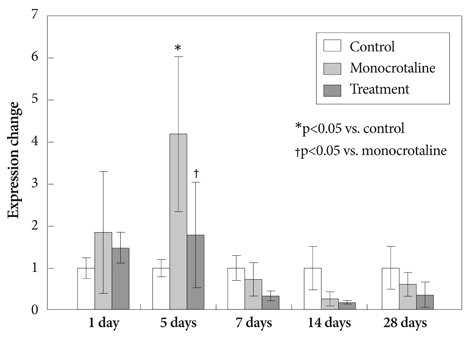
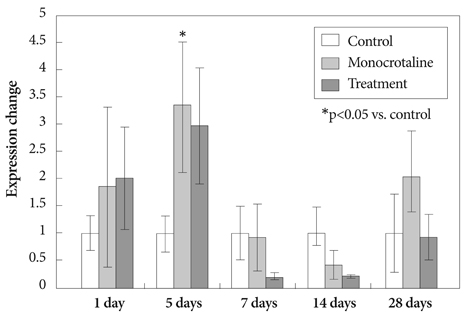
Serum ET-1 concentrations in the MCT group were higher than the control group on day 28 and 42. Quantitative analysis of peripheral pulmonary arteries revealed that the increase in medial wall thickness after MCT injection was significantly attenuated in the bosentan group on day 28 and 42. In addition, the increase in the number of intra-acinar muscular arteries after MCT injection was reduced by bosentan on day 14, 28 and 42. The levels of ET-1 and ERA gene expression were significantly increased in the MCT group compared with control group on day 5, and bosentan decreased the expression of ET-1 on day 5.
CONCLUSION
ET-1 contributes to the progression of cardiopulmonary pathology in rats with MCT-induced pulmonary hypertension. Administration of bosentan reduced ET-1 gene expression in MCT-induced pulmonary hypertension in rats.
MeSH Terms
Figure
Cited by 4 articles
-
Microarray Analysis in Pulmonary Hypertensive Rat Heart after Simvastatin Treatment
Yi Kyung Kim, Kwan Chang Kim, Young Mi Hong
Ewha Med J. 2018;41(3):53-62. doi: 10.12771/emj.2018.41.3.53.Effect of Ambrisentan Therapy on the Expression of Endothelin Receptor, Endothelial Nitric Oxide Synthase and NADPH Oxidase 4 in Monocrotaline-induced Pulmonary Arterial Hypertension Rat Model
Hyeryon Lee, Arim Yeom, Kwan Chang Kim, Young Mi Hong
Korean Circ J. 2019;49(9):866-876. doi: 10.4070/kcj.2019.0006.Effects of Bosentan Treatment on Angiotensin Converting Enzyme in Monocrotaline Induced Pulmonary Hypertension Rats
Sung Jin Kim, Ji Hae Cha, Hae Ryun Lee, Young Mi Hong
J Korean Soc Hypertens. 2011;17(1):28-36. doi: 10.5646/jksh.2011.17.1.28.Novel Genome-Wide Interactions Mediated via
BOLL andEDNRA Polymorphisms in Intracranial Aneurysm
Eun Pyo Hong, Dong Hyuk Youn, Bong Jun Kim, Jae Jun Lee, Sehyeon Nam, Hyojong Yoo, Heung Cheol Kim, Jong Kook Rhim, Jeong Jin Park, Jin Pyeong Jeon
J Korean Neurosurg Soc. 2023;66(4):409-417. doi: 10.3340/jkns.2022.0026.
Reference
-
1. Kim HW, Kim , Je HG, et al. Pulmonary arterial hypertension in children: a single center experience. Korean Circ J. 2008. 38:644–650.2. Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988. 332:411–415.3. Jung JW. Pulmonary arterial hypertension of congenital heart diseases: from reversible pulmonary hypertension to Eisenmenger syndrome. Korean Circ J. 2007. 37:287–297.4. Cody RJ, Haas GJ, Binkley PF, Capers Q, Kelley R. Plasma endothelin correlates with the extent of pulmonary hypertension in patients with chronic congestive heart failure. Circulation. 1992. 85:504–509.5. Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993. 328:1732–1739.6. Teerlink JR, Loffler BM, Hess P, Maire JP, Clozel M, Clozel JP. Role of endothelin in the maintenance of blood pressure in conscious rats with chronic heart failure: acute effects of the endothelin receptor antagonist Ro 47-0203 (bosentan). Circulation. 1994. 90:2510–2518.7. Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med. 2007. 28:23–42. vii8. Sakurai T, Yanagisawa M, Masaki T. Molecular characterization of endothelin receptors. Trends Pharmacol Sci. 1992. 13:103–108.9. Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990. 348:730–732.10. Zamora MA, Dempsey EC, Walchak SJ, Stelzner TJ. BQ123, an ETA receptor antagonist, inhibits endothelin-1-mediated proliferation of human pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol. 1993. 9:429–433.11. Vane J. Endothelins come home to roost. Nature. 1990. 348:673.12. de Nucci G, Thomas R, D'Orleans-Juste P, et al. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988. 85:9797–9800.13. Dupuis J, Goresky CA, Stewart DJ. Pulmonary removal and production of endothelin in the anesthetized dog. J Appl Physiol. 1994. 76:694–700.14. Dupuis J, Goresky CA, Fournier A. Pulmonary clearance of circulating endothelin1 in dogs in vivo: exclusive role of ETB receptors. J Appl Physiol. 1996. 81:1510–1515.15. Sumner MJ, Cannon TR, Mundin JW, White DG, Watts IS. Endothelin ETA and ETB receptors mediate vascular smooth muscle contraction. Br J Pharmacol. 1992. 107:858–860.16. Todd L, Mullen M, Olley PM, Rabinovitch M. Pulmonary toxicity of monocrotaline differs at critical periods of lung development. Pediatr Res. 1985. 19:731–737.17. Sohn DW, Kim HK, Kim MA, et al. Beneficial and adverse effects of bosentan treatment in Korean patients with pulmonary artery hypertension. Korean Circ J. 2009. 39:105–110.18. Channick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001. 358:1119–1123.19. Clozel M, Hess P, Rey M, Iglarz M, Binkert C, Qiu C. Bosentan, sildenafil, and their combination in the monocrotaline model of pulmonary hypertension in rats. Exp Biol Med. 2006. 231:967–973.20. Kim H, Yung GL, Marsh JJ, et al. Endothelin mediates pulmonary vascular remodelling in a canine model of chronic embolic pulmonary hypertension. Eur Respir J. 2000. 15:640–648.21. Morice AH, Mulrennan S, Clark A. Combination therapy with bosentan and phosphodiesterase-5 inhibitor in pulmonary arterial hypertension. Eur Respir J. 2005. 26:180–181.22. Lim KA, Shin JY, Cho SH, Kim KW, Han JJ, Hong YM. Effect of endothelin receptor blokade on monocrotaline-induced pulmonary hypertension in rats. Korean J Pediatr. 2009. 52:689–695.23. Miyauchi T, Yorikane R, Sakai S, et al. Contribution of endogenous endothelin-1 to the progression of cardiopulmonary alterations in rats with monocrotalin-induced pulmonary hypertension. Circ Res. 1993. 73:887–897.24. Itoh T, Nagaya N, Fujii T, et al. A combination of oral sildenafil and beraprost ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med. 2004. 169:34–38.25. Prie S, Leung TK, Cernacek P, Ryan JW, Dupuis J. The orally active ET(A) receptor antagonist (+)-(S)-2-(4,6-dimethoxy-pyrimidin-2-yloxy)-3-methoxy-3,3-diphenyl-propionic acid (LU 135252) prevents the development of pulmonary hypertension and endothelial metabolic dysfunction in monocrotaline-treated rats. J Pharmacol Exp Ther. 1997. 282:1312–1318.26. Schermuly RT, Kreisselmeier KP, Ghofrani HA, et al. Chronic sildenafil treatment inhibits monocrotaline-induced pulmonary hypertension in rats. Am J Respir Crit Care Med. 2004. 169:39–45.27. Park HK, Park SJ, Kim CS, Paek YW, Lee JU, Lee WJ. Enhanced gene expression of renin-angiotensin system, TGF-beta1, endothelin-1 and nitric oxide synthase in right-ventricular hypertrophy. Pharmacol Res. 2001. 43:265–273.28. Shiba R, Yanagisawa M, Miyauchi T, et al. Elimination of intravenously injected endothelin-1 from the circulation of the rat. J Cardiovasc Pharmacol. 1989. 13:Suppl 5. S98–S101. discussion S102.29. Weber C, Schmitt R, Birnboeck H, et al. Pharmacokinetics and pharmacodynamics of the endothelin-receptor antagonist bosentan in healthy human subjects. Clin Pharmacol Ther. 1996. 60:124–137.30. Loffler BM, Breu V, Clozel M. Effect of different endothelin receptor antagonists and of the novel non-peptide antagonist Ro 46-2005 on endothelin levels in rat plasma. FEBS Lett. 1993. 333:108–110.31. Ichikawa KI, Hidai C, Okuda C, et al. Endogenous endothelin-1 mediates cardiac hypertrophy and switching of myosin heavy chain gene expression in rat ventricular myocardium. J Am Coll Cardiol. 1996. 27:1286–1291.32. Dai ZK, Tan MS, Chai CY, et al. Effects of sildenafil on pulmonary hypertension and levels of ET-1, eNOS, and cGMP in aorta-banded rats. Exp Biol Med. 2006. 231:942–947.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of endothelin receptor blockade on monocrotaline-induced pulmonary hypertension in rats
- An inhibitory effect of tumor necrosis factor-alpha antagonist to gene expression in monocrotaline-induced pulmonary hypertensive rats model
- Effect of Ambrisentan Therapy on the Expression of Endothelin Receptor, Endothelial Nitric Oxide Synthase and NADPH Oxidase 4 in Monocrotaline-induced Pulmonary Arterial Hypertension Rat Model
- Bosentan Attenuates Compensatory Left Ventricular Hypertrophy Induced by Aortocaval Fistula in Rats
- Effects of Bosentan Treatment on Angiotensin Converting Enzyme in Monocrotaline Induced Pulmonary Hypertension Rats