Korean Diabetes J.
2008 Apr;32(2):112-120. 10.4093/kdj.2008.32.2.112.
The Effect of Chronic High Glucose Concentration on Endoplasmic Reticulum Stress in INS-1 Cells
- Affiliations
-
- 1Department of Internal Medicine, Keimyung University School of Medicine, Korea.
- 2Department of Internal Medicine, Kyungpook National University School of Medicine, Korea.
- KMID: 2222507
- DOI: http://doi.org/10.4093/kdj.2008.32.2.112
Abstract
-
BACKGROUND: The highly developed endoplasmic reticulum (ER) structure is one of the characteristic features of pancreatic beta-cells. Recent study showed that ER stress causes beta-cell dysfunction. However, little is known about the effects of high glucose concentration on induction of ER stress in pancreatic beta-cells. Therefore, this study was designed to evaluate whether exposure of high glucose concentration in rat insulinoma cell line, INS-1 cell induces ER stress and whether ER stress decreases insulin gene expression.
METHODS
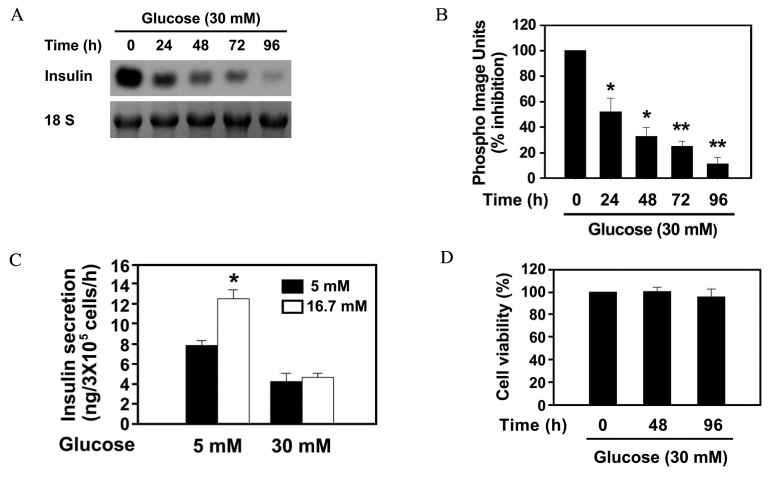
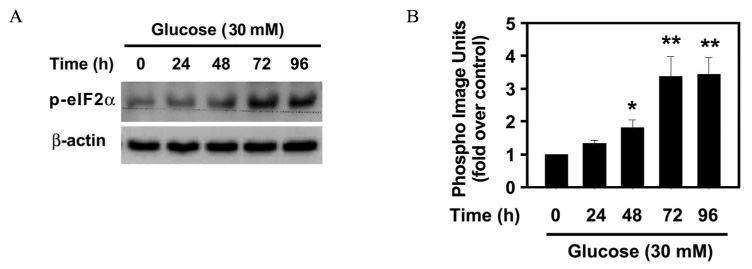
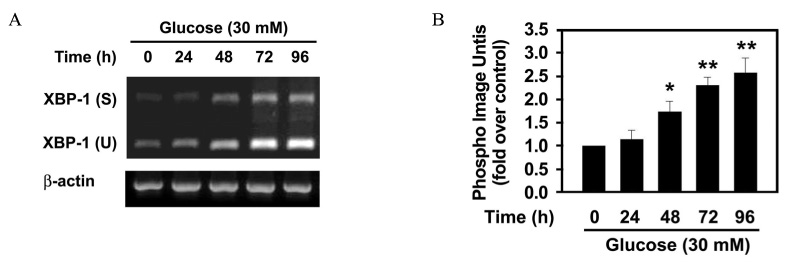
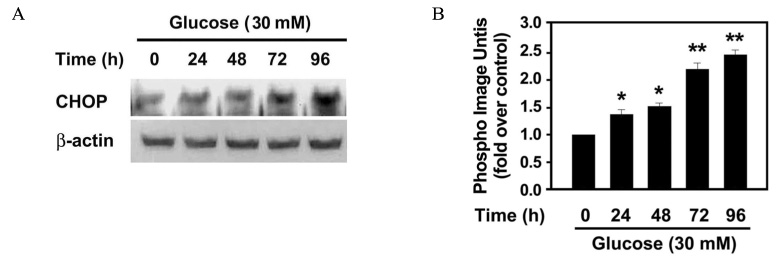
The effect of 30 mM glucose on insulin expression and secretion in INS-1 cells was evaluated by Northern blot analysis and glucose-stimulated insulin secretion (GSIS). Cell viability was evaluated by XTT assay. The effect of 30 mM glucose on phosphorylation of eIF2alpha and CHOP expression, which are markers of ER stress were evaluated by Western blot analysis. RT-PCR analysis was performed to determine whether high glucose concentration induces XBP-1 splicing. To investigate whether ER stress decreases insulin gene expression, the effect of tunicamycin on insulin mRNA expression was evaluated by Northern blot analysis.
RESULTS
The prolonged exposure of INS-1 cells with the 30 mM glucose concentration decreased insulin mRNA expression in a time dependent manner and impaired GSIS while did not influence on cell viability. 30 mM glucose increased phosphorylation of eIF2alpha, XBP-1 splicing and CHOP expression in INS-1 cells. Tunicamycin-treated INS-1 increased XBP-1 splicing and decreased insulin mRNA expression in a dose dependent manner.
CONCLUSION
This study showed that prolonged exposure of INS-1 with high glucose concentration induces ER stress and ER stress decreases insulin gene expression. Further studies about underlying molecular mechanism by which ER stress induces beta-cell dysfunction are needed.
MeSH Terms
Figure
Reference
-
1. Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005. 74:739–789.
Article2. Oyadomari S, Araki E, Mori M. Endoplasmic reticulum stress mediated apoptosis in pancreatic β-cell. Apoptosis. 2002. 7:335–345.3. Mori K. Tripartitie management of unfolded proteins in the endoplasmic reticulum. Cell. 2000. 101:451–454.4. Kaufman RJ, Scheuner D, Schroder M, Shen X, Lee K, Liu CY, Arnold SM. The unfolded protein response in nutrient sensing and differentiation. Nature Reviews Molecular Cell Biology. 2002. 3:411–421.
Article5. Kenneth LB. Principles and practice of endocrinology and metabolism. 2001. 3rd ed. Philadelphia: W.B. Sauders;1296.6. Dodson G, Steiner D. The role of assembly in insulin's biosynthesis. Curr Opin Struct Biol. 1998. 8:189–194.
Article7. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004. 306:457–461.
Article8. Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in Perk-/- mice reveals a role for translational control in secretory cell survival. Molecular Cell. 2001. 7:1153–1163.
Article9. Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002. 109:525–532.
Article10. Park KG, Lee KM, Seo HY, Suh JH, Kim HS, Wang L, Won KC, Lee HW, Park JY, Lee KU, Kim JG, Kim BW, Choi HS, Lee IK. Glucotoxicity in the INS-1 rat insulinoma cell line is mediated by the orphan nuclear receptor small heterodimer partner. Diabetes. 2007. 56:431–437.
Article11. Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007. 50:752–763.
Article12. Oydomari S, Takeda K, Takiguchi M, Gotoh T, Matsumoto M, Wada I, Akira S, Araki E, Mori M. Nitric oxide-induced apoptosis in pancreatic β cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci USA. 2001. 98:10845–10850.13. Cardozo AK, Ortis F, Storling JS, Feng YM, Rasschaert J, Tonnesen MT, Eylen FV, Mandrup-Poulsen T, Herchuelz A, Eizirik DL. Cytokines Downregulate the Sarcoendoplasmic Reticulum Pump Ca2ATPase 2b and Deplete Endoplasmic Reticulum Ca2, Leading to Induction of Endoplasmic Reticulum Stress in Pancreatic β-Cells. Diabetes. 2005. 54:452–461.14. Wang H, Kouri G, Wolheim CB. ER stress and SREBP-1 activation are implicated in β-cell glucolipotoxicity. J Cell Sci. 2005. 118:3905–3915.
Article15. Xue X, Piao JH, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H, Nakano H. Tumor necrosis factor alpha (TNF alpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNF alpha. J Biol Chem. 2005. 280:33917–33925.16. Hsieh YH, Su IJ, Lei HY, Lai MD, Chang WW, Huang W. Differential endoplasmic reticulum stress signaling pathways mediated by iNOS. Biochem Biophys Res Commun. 2007. 359:643–648.
Article17. Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell metabolism. 2006. 4:245–254.
Article18. Pirot P, Naamane N, Libert F, Magnusson NE, Orntoft TF, Cardozo AK, Eizirik DL. Global profiling of genes modified by endoplasmic reticulum stress in pancreatic beta cells reveals the early degradation of insulin mRNAs. Diabetologia. 2007. 50:1006–1014.
Article19. Harding HP, Zhang Y, Bertolotti A, Zeng H, David R. Perk is essential for translational regulation and cell surival during the unfolded protein response. Molecular cell. 2000. 5:897–904.20. Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K. ATF6 activated by proteolysis binds in the presence of NF-Y(CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Molecular and Cellular Biology. 2000. 20:6755–6767.21. Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress response. EMBO. 1998. 19:5708–5717.22. Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998. 12:982–995.23. McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001. 21:1249–1259.
Article24. Araki E, Oyadomari S, Mori M. Impact of endoplasmic reticulum stress pathway on pancreatic β-cells and diabetes mellitus. Exp Biol Med. 2003. 228:1213–1217.
Article25. Mohanty S, Spinas GA, Maedler K, Zuellig RA, Lehmann R, Donath MY, Trüb T, Niessen M. Overexpression of IRS2 in isolated pancreatic islets causes proliferation and protects human beta-cells from hyperglycemia-induced apoptosis. Exp Cell Res. 2005. 1:68–78.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoplasmic Reticulum Stress and Diabetes
- The Effect of Tribbles-Related Protein 3 on ER Stress-Suppressed Insulin Gene Expression in INS-1 Cells
- New Insights into the Role of Endoplasmic Reticulum Stress in Breast Cancer Metastasis
- The Effects of Glyburide on Apoptosis and Endoplasmic Reticulum Stress in INS-1 Cells in a Glucolipotoxic Condition
- Low glibenclamide concentrations affect endoplasmic reticulum stress in INS-1 cells under glucotoxic or glucolipotoxic conditions