Korean Diabetes J.
2009 Jun;33(3):198-205. 10.4093/kdj.2009.33.3.198.
Nitric Oxide Increases Insulin Sensitivity in Skeletal Muscle by Improving Mitochondrial Function and Insulin Signaling
- Affiliations
-
- 1Department of Internal Medicine, University of Ulsan College of Medicine, Seoul, Korea. wjlee@amc.seoul.kr
- 2Asan Institute for Life Sciences, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2222419
- DOI: http://doi.org/10.4093/kdj.2009.33.3.198
Abstract
- BACKGROUND
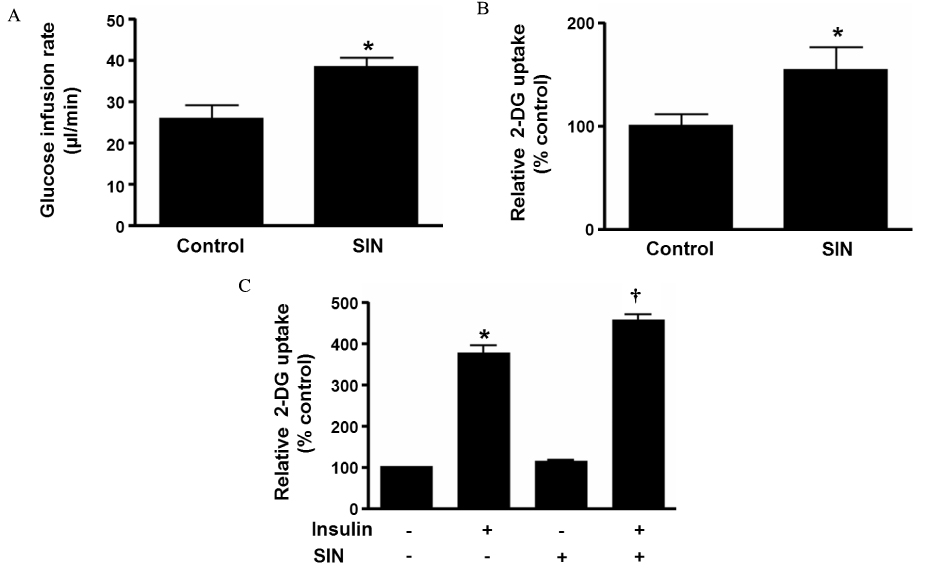
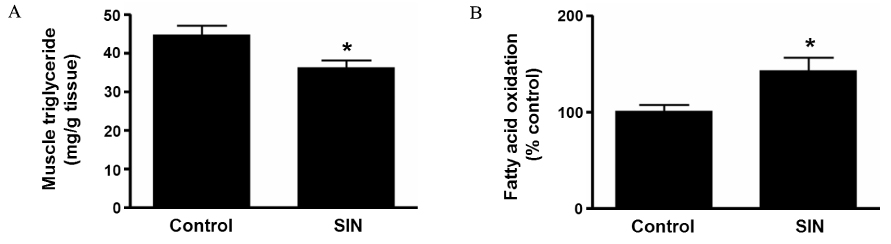
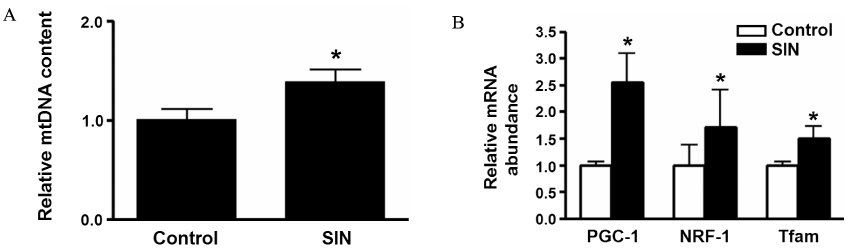
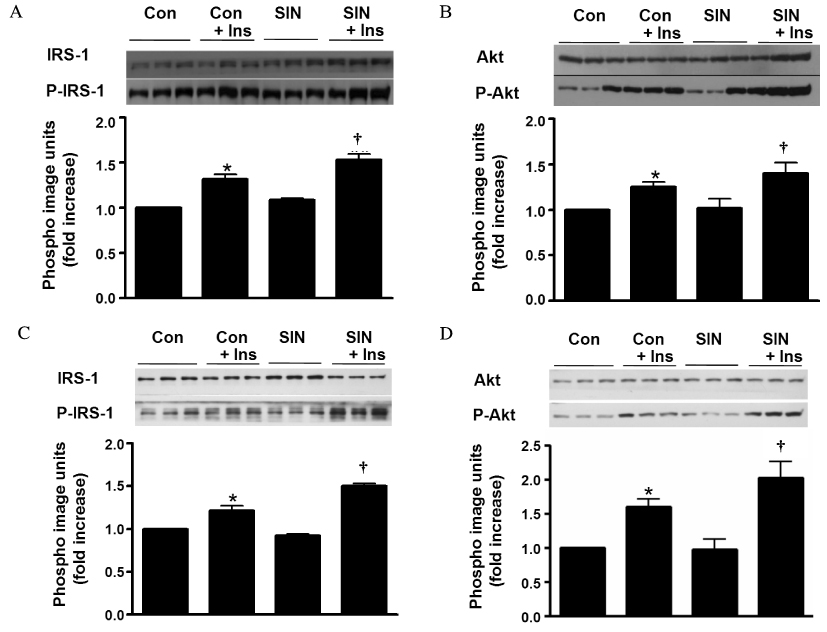
Accumulating evidence has suggested that nitric oxide (NO) is involved in the regulation of insulin sensitivity in skeletal muscle. Recent studies also suggested NO as an important molecule regulating mitochondrial biogenesis. This study examined the effect of the NO donor, 3-morpholinosydnonimine (SIN-1), on glucose metabolism in skeletal muscle and tested the hypothesis that NO's effect on glucose metabolism is mediated by its effect on mitochondrial function. METHODS: In Sprague-Dawley (SD) rats treated with SIN-1 for 4 weeks, insulin sensitivity was measured by a glucose clamp study. Triglyceride content and fatty acid oxidation were measured in the skeletal muscle. In addition, mitochondrial DNA content and mRNA expression of mitochondrial biogenesis markers were assessed by real-time polymerase chain reaction and expression of insulin receptor substrate (IRS)-1 and Akt were examined by Western blot analysis in skeletal muscle. In C2C12 cells, insulin sensitivity was measured by 2-deoxyglucose uptake and Western blot analysis was used to examine the expression of IRS-1 and Akt. RESULTS: SIN-1 improved insulin sensitivity in C2C12 cells and skeletal muscles of SD rats. In addition, SIN-1 decreased triglyceride content and increased fatty acid oxidation in skeletal muscle. Mitochondrial DNA contents and biogenesis in the skeletal muscle were increased by SIN-1 treatment. Moreover, SIN-1 increased the expression of phosphor-IRS-1 and phosphor-Akt in the skeletal muscle and muscle cells. CONCLUSION: Our results suggest that NO mediates glucose uptake in skeletal muscle both in vitro and in vivo by improving mitochondrial function and stimulating insulin signaling pathways.
MeSH Terms
-
Animals
Organelle Biogenesis
Blotting, Western
Deoxyglucose
DNA, Mitochondrial
Glucose
Glucose Clamp Technique
Humans
Insulin
Insulin Resistance
Mitochondria
Muscle Cells
Muscle, Skeletal
Muscles
Nitric Oxide
Rats
Real-Time Polymerase Chain Reaction
Receptor, Insulin
RNA, Messenger
Signal Transduction
Tissue Donors
DNA, Mitochondrial
Deoxyglucose
Glucose
Insulin
Nitric Oxide
RNA, Messenger
Receptor, Insulin
Figure
Reference
-
1. Lane P, Gross SS. Cell signaling by nitric oxide. Semin Nephrol. 1999. 19:215–229.2. Gross SS, Wolin MS. Nitric oxide: Pathophysiological mechanisms. Annu Rev Physiol. 1995. 57:737–769.3. Moncada S, Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med. 1993. 329:2002–2012.4. Kapur S, Bedard S, Marcotte B, Cote CH, Marette A. Expression of nitric oxide synthase in skeletal muscle: A novel role for nitric oxide as a modulator of insulin action. Diabetes. 1997. 46:1691–1700.5. Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994. 372:546–548.6. Balon TW, Nadler JL. Evidence that nitric oxide increases glucose transport in skeletal muscle. J Appl Physiol. 1997. 82:359–363.7. Young ME, Radda GK, Leighton B. Nitric oxide stimulates glucose transport and metabolism in rat skeletal muscle in vitro. Biochem J. 1997. 322(Pt 1):223–228.8. Etgen GJ Jr, Fryburg DA, Gibbs EM. Nitric oxide stimulates skeletal muscle glucose transport through a calcium/contraction- and phosphatidylinositol-3-kinase-independent pathway. Diabetes. 1997. 46:1915–1919.9. Lira VA, Soltow QA, Long JH, Betters JL, Sellman JE, Criswell DS. Nitric oxide increases GLUT4 expression and regulates AMPK signaling in skeletal muscle. Am J Physiol Endocrinol Metab. 2007. 293:E1062–E1068.10. Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B, Scherrer U. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001. 104:342–345.11. Shankar RR, Wu Y, Shen HQ, Zhu JS, Baron AD. Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Diabetes. 2000. 49:684–687.12. Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005. 307:384–387.13. Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: The role of endogenous nitric oxide. Science. 2003. 299:896–899.14. Lee WJ, Song KH, Koh EH, Won JC, Kim HS, Park HS, Kim MS, Kim SW, Lee KU, Park JY. Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem Biophys Res Commun. 2005. 332:885–891.15. Kase ET, Wensaas AJ, Aas V, Hojlund K, Levin K, Thoresen GH, Beck-Nielsen H, Rustan AC, Gaster M. Skeletal muscle lipid accumulation in type 2 diabetes may involve the liver x receptor pathway. Diabetes. 2005. 54:1108–1115.16. Cha SH, Hu Z, Chohnan S, Lane MD. Inhibition of hypothalamic fatty acid synthase triggers rapid activation of fatty acid oxidation in skeletal muscle. Proc Natl Acad Sci U S A. 2005. 102:14557–14562.17. Bogacka I, Xie H, Bray GA, Smith SR. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005. 54:1392–1399.18. Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, Pisconti A, Brunelli S, Cardile A, Francolini M, Cantoni O, Carruba MO, Moncada S, Clementi E. Mitochondrial biogenesis by no yields functionally active mitochondria in mammals. Proc Natl Acad Sci U S A. 2004. 101:16507–16512.19. Kobzik L, Stringer B, Balligand JL, Reid MB, Stamler JS. Endothelial type nitric oxide synthase in skeletal muscle fibers: Mitochondrial relationships. Biochem Biophys Res Commun. 1995. 211:375–381.20. Higaki Y, Hirshman MF, Fujii N, Goodyear LJ. Nitric oxide increases glucose uptake through a mechanism that is distinct from the insulin and contraction pathways in rat skeletal muscle. Diabetes. 2001. 50:241–247.21. Roy D, Perreault M, Marette A. Insulin stimulation of glucose uptake in skeletal muscles and adipose tissues in vivo is no dependent. Am J Physiol. 1998. 274:E692–E699.22. Baron AD. The coupling of glucose metabolism and perfusion in human skeletal muscle. The potential role of endothelium-derived nitric oxide. Diabetes. 1996. 45:suppl 1. S105–S109.23. Shankar R, Zhu JS, Ladd B, Henry D, Shen HQ, Baron AD. Central nervous system nitric oxide synthase activity regulates insulin secretion and insulin action. J Clin Invest. 1998. 102:1403–1412.24. Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G. Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest. 1995. 96:786–792.25. Higaki Y, Wojtaszewski JF, Hirshman MF, Withers DJ, Towery H, White MF, Goodyear LJ. Insulin receptor substrate-2 is not necessary for insulin- and exercise-stimulated glucose transport in skeletal muscle. J Biol Chem. 1999. 274:20791–20795.26. Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006. 55:suppl 2. S9–S15.27. Park SY, Lee W. The depletion of cellular mitochondrial DNA causes insulin resistance through the alteration of insulin receptor substrate-1 in rat myocytes. Diabetes Res Clin Pract. 2007. 77:suppl 1. S165–S171.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The relationship between muscle mitochondrial nutritional overloading and insulin resistance
- AMPK and Exercise: Glucose Uptake and Insulin Sensitivity
- Insulin Resistance and Intracellular Thyroid Hormone Dysfunction
- Insulin Resistance and Erectile Dysfunction
- Skeletal Muscle Mitochondria and Insulin Resistance: The Role of Exercise