Electrolyte Blood Press.
2013 Dec;11(2):46-52. 10.5049/EBP.2013.11.2.46.
Obesity Associated Hypertension: New Insights into Mechanism
- Affiliations
-
- 1Division of Nephrology, Department of Internal Medicine, Medical College of Korea University, Ansan Hospital, Ansan-city, Gyeonggi, Korea. starch70@korea.ac.kr
- KMID: 2168400
- DOI: http://doi.org/10.5049/EBP.2013.11.2.46
Abstract
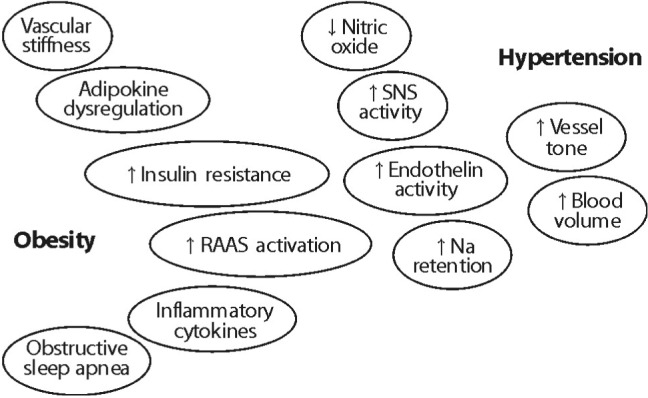
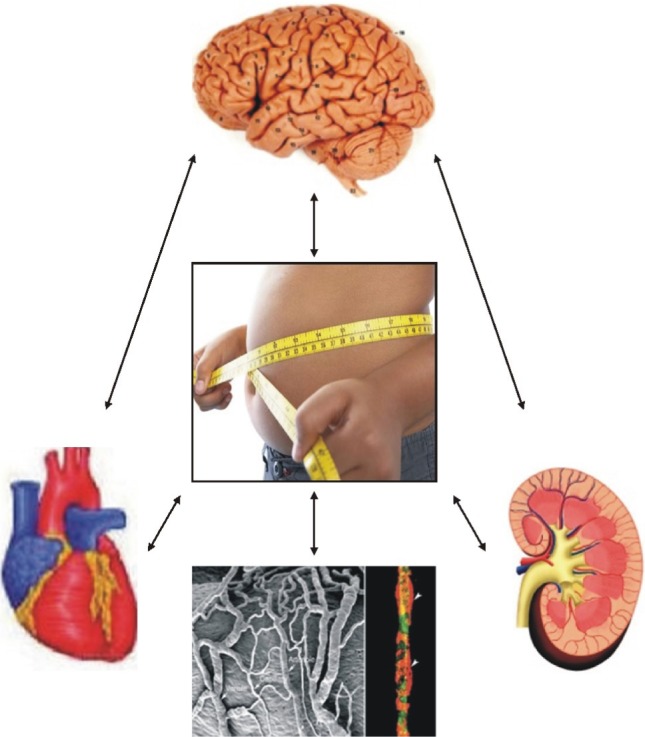
- With excess nutrition, the burden of obesity is a growing problem worldwide. The imbalance between energy intake and expenditure leads to variable disorders as all major risk factors for cardiovascular disease. There are many hypothetical mechanisms to explain obesity-associated hypertension. Activation of the RAAS is a key contributing factor in obesity. Particularly, the RAAS in adipose tissue plays a crucial role in adipose tissue dysfunction and obesity-induced inflammation. The phenotypic changes of adipocytes occur into hypertrophy and an inflammatory response in an autocrine and paracrine manner to impair adipocyte function, including insulin signaling pathway. Adipose tissue produce and secretes several molecules such as leptin, resistin, adiponectin, and visfatin, as well as cytokines such as TNF-alpha, IL-6, MCP-1, and IL-1. These adipokines are stimulated via the intracellular signaling pathways that regulate inflammation of adipose tissue. Inflammation and oxidative stress in adipose tissue are important to interact with the microvascular endothelium in the mechanisms of obesity-associated hypertension. Increased microvascular resistance raises blood pressure. Therefore, a regulatory link between microvascular and perivascular adipose tissue inflammation and adipokine synthesis are provided to explain the mechanism of obesity-associated hypertension.
Keyword
MeSH Terms
-
Adipocytes
Adipokines
Adiponectin
Adipose Tissue
Blood Pressure
Cardiovascular Diseases
Cytokines
Endothelium
Energy Intake
Health Expenditures
Hypertension*
Hypertrophy
Inflammation
Insulin
Insulin Resistance
Interleukin-1
Interleukin-6
Leptin
Nicotinamide Phosphoribosyltransferase
Obesity*
Oxidative Stress
Resistin
Risk Factors
Tumor Necrosis Factor-alpha
Adipokines
Adiponectin
Cytokines
Insulin
Interleukin-1
Interleukin-6
Leptin
Nicotinamide Phosphoribosyltransferase
Resistin
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Kramer H. Obesity and chronic kidney disease. Contrib Nephrol. 2006; 151:1–18. PMID: 16929130.
Article2. Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of The Obesity Society and the American Society of Hypertension. J Clin Hypertens (Greenwich). 2013; 15:14–33. PMID: 23282121.3. Aghamohammadzadeh R, Heagerty AM. Obesity-related hypertension: epidemiology, pathophysiology, treatments, and the contribution of perivascular adipose tissue. Ann Med. 2012; 44(Suppl 1):S74–S84. PMID: 22713152.
Article4. Westerbacka J, Vehkavaara S, Bergholm R, Wilkinson I, Cockcroft J, Yki-Jarvinen H. Marked resistance of the ability of insulin to decrease arterial stiffness characterizes human obesity. Diabetes. 1999; 48:821–827. PMID: 10102699.
Article5. Matsuzawa Y. The metabolic syndrome and adipocytokines. FEBS Lett. 2006; 580:2917–2921. PMID: 16674947.
Article6. Ferrante AW Jr. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007; 262:408–414. PMID: 17875176.
Article7. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993; 259:87–91. PMID: 7678183.8. Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996; 271:665–668. PMID: 8571133.9. Schaffler A, Scholmerich J. Innate immunity and adipose tissue biology. Trends Immunol. 2010; 31:228–235. PMID: 20434953.10. Majdalawieh A, Ro HS. Regulation of Ikappa Balpha function and NF-kappaB signaling: AEBP1 is a novel proinflammatory mediator in macrophages. Mediators Inflamm. 2010; 2010:823821. PMID: 20396415.11. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009; 15:914–920. PMID: 19633658.12. Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009; 15:930–939. PMID: 19633656.
Article13. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007; 117:175–184. PMID: 17200717.
Article14. Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005; 11:183–190. PMID: 15685173.15. Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010; 59:1648–1656. PMID: 20357360.
Article16. Zeyda M, Gollinger K, Kriehuber E, Kiefer FW, Neuhofer A, Stulnig TM. Newly identified adipose tissue macrophage populations in obesity with distinct chemokine and chemokine receptor expression. Int J Obes (Lond). 2010; 34:1684–1694. PMID: 20514049.
Article17. Hansson L, Lindholm LH, Niskanen L, Lanke J, Hedner T, Niklason A, et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet. 1999; 353:611–616. PMID: 10030325.
Article18. Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000; 342:145–153. PMID: 10639539.
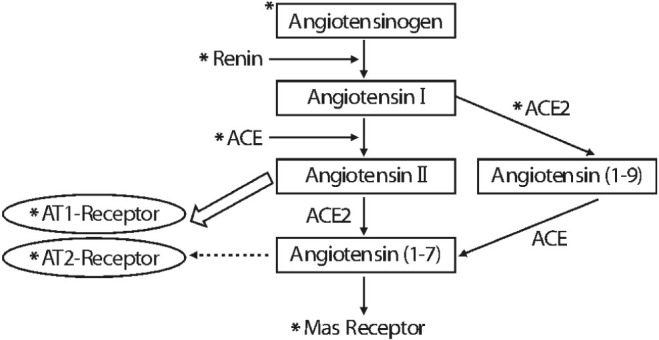
Article19. Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002; 359:995–1003. PMID: 11937178.20. Engeli S. Role of the renin-angiotensin-aldosterone system in the metabolic syndrome. Contrib Nephrol. 2006; 151:122–134. PMID: 16929137.21. Engeli S, Negrel R, Sharma AM. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension. 2000; 35:1270–1277. PMID: 10856276.
Article22. Campbell DJ, Habener JF. Cellular localization of angiotensinogen gene expression in brown adipose tissue and mesentery: quantification of messenger ribonucleic acid abundance using hybridization in situ. Endocrinology. 1987; 121:1616–1626. PMID: 3665835.
Article23. Massiera F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001; 15:2727–2729. PMID: 11606482.24. Hainault I, Nebout G, Turban S, Ardouin B, Ferre P, Quignard-Boulange A. Adipose tissue-specific increase in angiotensinogen expression and secretion in the obese (fa/fa) Zucker rat. Am J Physiol Endocrinol Metab. 2002; 282:E59–E66. PMID: 11739084.25. Van Harmelen V, Ariapart P, Hoffstedt J, Lundkvist I, Bringman S, Arner P. Increased adipose angiotensinogen gene expression in human obesity. Obes Res. 2000; 8:337–341. PMID: 10933310.
Article26. Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, et al. Weight loss and the renin-angio tensin-aldosterone system. Hypertension. 2005; 45:356–362. PMID: 15630041.27. Thatcher S, Yiannikouris F, Gupte M, Cassis L. The adipose renin-angiotensin system: role in cardiovascular disease. Mol Cell Endocrinol. 2009; 302:111–117. PMID: 19418627.
Article28. Yvan-Charvet L, Even P, Bloch-Faure M, Guerre-Millo M, Moustaid-Moussa N, Ferre P, et al. Deletion of the angiotensin type 2 receptor (AT2R) reduces adipose cell size and protects from diet-induced obesity and insulin resistance. Diabetes. 2005; 54:991–999. PMID: 15793237.
Article29. Kouyama R, Suganami T, Nishida J, Tanaka M, Toyoda T, Kiso M, et al. Attenuation of diet-induced weight gain and adiposity through increased energy expenditure in mice lacking angiotensin II type 1a receptor. Endocrinology. 2005; 146:3481–3489. PMID: 15878965.
Article30. Santos SH, Fernandes LR, Mario EG, Ferreira AV, Porto LC, Alvarez-Leite JI, et al. Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes. 2008; 57:340–347. PMID: 18025412.
Article31. Takahashi N, Li F, Hua K, Deng J, Wang CH, Bowers RR, et al. Increased energy expenditure, dietary fat wasting, and resistance to diet-induced obesity in mice lacking renin. Cell Metab. 2007; 6:506–512. PMID: 18054319.
Article32. Jayasooriya AP, Mathai ML, Walker LL, Begg DP, Denton DA, Cameron-Smith D, et al. Mice lacking angiotensin-converting enzyme have increased energy expenditure, with reduced fat mass and improved glucose clearance. Proc Natl Acad Sci USA. 2008; 105:6531–6536. PMID: 18443281.
Article33. Yvan-Charvet L, Quignard-Boulange A. Role of adipose tissue renin-angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int. 2011; 79:162–168. PMID: 20944545.
Article34. Lee MH, Song HK, Ko GJ, Kang YS, Han SY, Han KH, et al. Angiotensin receptor blockers improve insulin resistance in type 2 diabetic rats by modulating adipose tissue. Kidney Int. 2008; 74:890–900. PMID: 18596725.
Article35. Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011; 57:1511–1522. PMID: 21453829.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Obesity Type on Diabetes and Hypertension in Obese Korean Adults
- The Effects of Abdominal Obesity on the Increased Prevalence Rate of Hypertension and Diabetes Mellitus in Benign Prostatic Hyperplasia Patients
- The Influence of Obesity and Metabolic Health on Vascular Health
- A Review of Childhood Obesity
- Effects of Abdominal Obesity and Risk Drinking on the Hypertension Risk in Korean Adults