Allergy Asthma Immunol Res.
2010 Jul;2(3):199-205. 10.4168/aair.2010.2.3.199.
The Effects of Lactobacillus rhamnosus on the Prevention of Asthma in a Murine Model
- Affiliations
-
- 1Department of Pediatrics, Childhood Asthma Atopy Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sjhong@amc.seoul.kr
- 2Asan Institute for Life Sciences, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2133809
- DOI: http://doi.org/10.4168/aair.2010.2.3.199
Abstract
- PURPOSE
Lactobacilli are probiotic bacteria that are effective in the management of allergic diseases or gastroenteritis. It is hypothesized that such probiotics have immunoregulatory properties and promote mucosal tolerance. Our goal was to investigate whether Lactobacillus casei rhamnosus Lcr35 could inhibit airway inflammation in an ovalbumin (OVA)-induced murine model of asthma.
METHODS
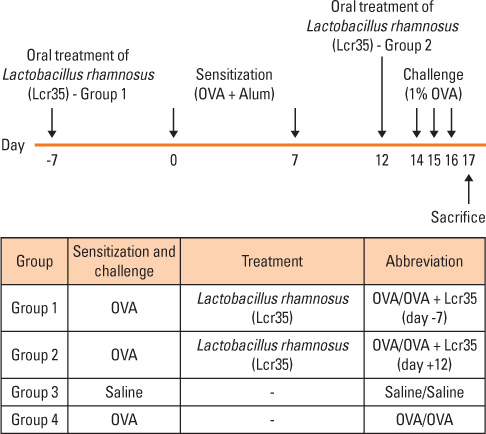
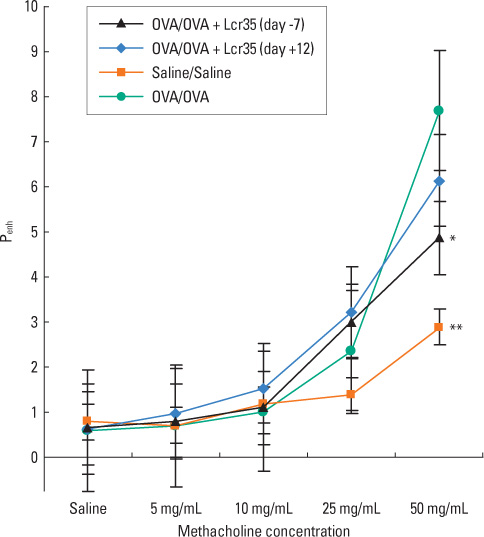
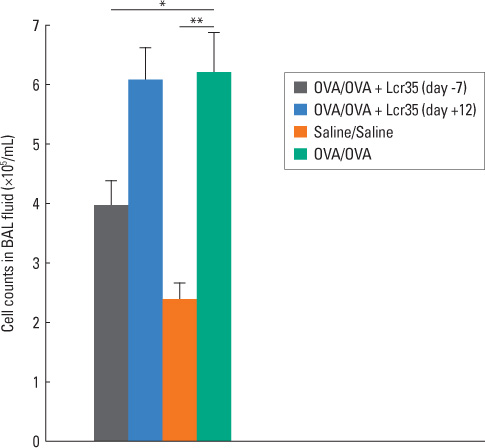
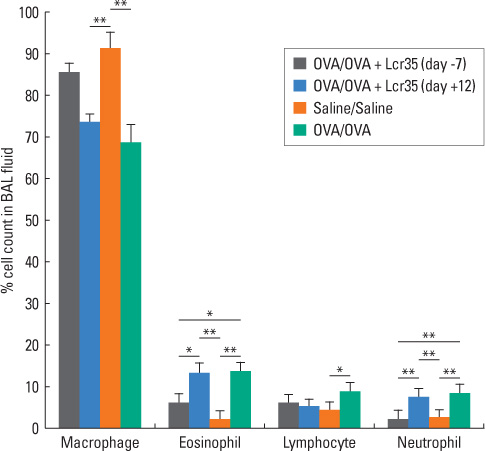
BALB/c mice aged 6 weeks were used in the present study. Lactobacillus casei rhamnosus Lcr35 was administered daily, starting 1 week prior to the first OVA sensitization (group 1) and 2 days before the first 1% OVA airway challenge (group 2). Mice that received only saline at both sensitization and airway challenge time points were used as negative controls (group 3), and those that had OVA-induced asthma were used as positive controls (group 4). Airway responsiveness to methacholine was assessed, and bronchoalveolar lavage (BAL) was performed. At the endpoint of the study, total IgE as well as OVA-specific IgE, IgG1 and IgG2a in serum was measured by enzyme-linked immunosorbent assay. Lung pathology was also evaluated.
RESULTS
Airway hyperresponsiveness, total cell counts and the proportion of eosinophils in BAL fluid were significantly decreased in group 1 compared with group 4 (P<0.05). Total serum IgE levels were also significantly decreased in group 1 compared with group 4. Serum levels of OVA-specific IgE, IgG1 and IgG(2a) were not significantly influenced by treatment with Lcr35. There was significantly less peribronchial and perivascular infiltration of inflammatory cells in group 1 compared with group 4; however, there were no significant differences in methacholine challenge, BAL, serology or histology between groups 2 and 4.
CONCLUSIONS
Oral treatment with Lcr35 prior to sensitization can attenuate airway inflammation and hyperreactivity in a mouse model of allergic airway inflammation. These results suggest that Lcr35 may have potential for preventing asthma.
MeSH Terms
-
Aged
Animals
Asthma
Bacteria
Bronchoalveolar Lavage
Cell Count
Disease Models, Animal
Enzyme-Linked Immunosorbent Assay
Eosinophils
Gastroenteritis
Humans
Immunoglobulin E
Immunoglobulin G
Inflammation
Lactobacillus
Lactobacillus casei
Lactobacillus rhamnosus
Lung
Methacholine Chloride
Mice
Ovalbumin
Ovum
Primary Prevention
Probiotics
Immunoglobulin E
Immunoglobulin G
Methacholine Chloride
Ovalbumin
Figure
Reference
-
1. Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992. 326:298–304.2. Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow JC Jr. Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992. 146:109–115.3. Del Prete GF, De Carli M, D'Elios MM, Maestrelli P, Ricci M, Fabbri L, Romagnani S. Allergen exposure induces the activation of allergen-specific Th2 cells in the airway mucosa of patients with allergic respiratory disorders. Eur J Immunol. 1993. 23:1445–1449.4. Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, Young IG, Vadas MA. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988. 167:219–224.5. Wang JM, Rambaldi A, Biondi A, Chen ZG, Sanderson CJ, Mantovani A. Recombinant human interleukin 5 is a selective eosinophil chemoattractant. Eur J Immunol. 1989. 19:701–705.6. Finkelman FD, Katona IM, Urban JF Jr, Holmes J, Ohara J, Tung AS, Sample JV, Paul WE. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988. 141:2335–2341.7. Check W. Innate immunity depends on Toll-like receptors. ASM News. 2004. 70:317–322.8. Boman HG. Antibacterial peptides: basic facts and emerging concepts. J Intern Med. 2003. 254:197–215.9. Abreu MT, Arditi M. Innate immunity and toll-like receptors: clinical implications of basic science research. J Pediatr. 2004. 144:421–429.10. Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002. 296:490–494.11. Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002. 347:911–920.12. Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest. 1999. 103:175–183.13. Akbari O, Stock P, DeKruyff RH, Umetsu DT. Role of regulatory T cells in allergy and asthma. Curr Opin Immunol. 2003. 15:627–633.14. Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol. 2001. 1:69–75.15. Kalliomaki M, Isolauri E. Role of intestinal flora in the development of allergy. Curr Opin Allergy Clin Immunol. 2003. 3:15–20.16. Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989. 66:365–378.17. Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001. 357:1076–1079.18. Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. 2003. 361:1869–1871.19. Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clin Exp Allergy. 2000. 30:1604–1610.20. Feleszko W, Jaworska J, Rha RD, Steinhausen S, Avagyan A, Jaudszus A, Ahrens B, Groneberg DA, Wahn U, Hamelmann E. Probiotic-induced suppression of allergic sensitization and airway inflammationis associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 2007. 37:498–498.21. Forsythe P, Inman MD, Bienenstock J. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. Am J Respir Crit Care Med. 2007. 175:561–569.22. Neuhaus-Steinmetz U, Glaab T, Daser A, Braun A, Lommatzsch M, Herz U, Kips J, Alarie Y, Renz H. Sequential development of airway hyperresponsiveness and acute airway obstruction in a mouse model of allergic inflammation. Int Arch Allergy Immunol. 2000. 121:57–67.23. Hart AL, Lammers K, Brigidi P, Vitali B, Rizzello F, Gionchetti P, Campieri M, Kamm MA, Knight SC, Stagg AJ. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004. 53:1602–1609.24. von der Weid T, Bulliard C, Schiffrin EJ. Induction by a lactic acid bacterium of a population of CD4(+) T cells with low proliferativecapacity that produce transforming growth factor beta and interleukin-10. Clin Diagn Lab Immunol. 2001. 8:695–701.25. Pochard P, Gosset P, Grangette C, Andre C, Tonnel AB, Pestel J, Mercenier A. Lactic acid bacteria inhibit TH2 cytokine production by mononuclear cells from allergic patients. J Allergy Clin Immunol. 2002. 110:617–623.26. Smits HH, Engering A, van der Kleij D, de Jong EC, Schipper K, van Capel TM, Zaat BA, Yazdanbakhsh M, Wierenga EA, van Kooyk Y, Kapsenberg ML. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005. 115:1260–1267.27. Gill HS, Rutherfurd KJ, Prasad J, Gopal PK. Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019). Br J Nutr. 2000. 83:167–176.28. Rosenfeldt V, Benfeldt E, Nielsen SD, Michaelsen KF, Jeppesen DL, Valerius NH, Paerregaard A. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol. 2003. 111:389–395.29. Viljanen M, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, Kuitunen M. Probiotics in the treatment ofatopic eczema/dermatitis syndrome in infants: a double-blind placebo-controlled trial. Allergy. 2005. 60:494–500.30. Brouwer ML, Wolt-Plompen SA, Dubois AE, van der Heide S, Jansen DF, Hoijer MA, Kauffman HF, Duiverman EJ. No effects of probiotics on atopic dermatitis in infancy: a randomized placebo-controlled trial. Clin Exp Allergy. 2006. 36:899–906.31. Wheeler JG, Shema SJ, Bogle ML, Shirrell MA, Burks AW, Pittler A, Helm RM. Immune and clinical impact of Lactobacillus acidophilus on asthma. Ann Allergy Asthma Immunol. 1997. 79:229–233.32. Helin T, Haahtela S, Haahtela T. No effect of oral treatment with an intestinal bacterial strain, Lactobacillus rhamnosus (ATCC 53103), on birch-pollen allergy: a placebo-controlled double-blind study. Allergy. 2002. 57:243–246.33. Wang MF, Lin HC, Wang YY, Hsu CH. Treatment of perennial allergic rhinitis with lactic acid bacteria. Pediatr Allergy Immunol. 2004. 15:152–158.34. Vliagoftis H, Kouranos VD, Betsi GI, Falagas ME. Probiotics for the treatment of allergic rhinitis and asthma: systematic review of randomized controlled trials. Ann Allergy Asthma Immunol. 2008. 101:570–579.35. Kopp MV, Hennemuth I, Heinzmann A, Urbanek R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of Lactobacillus GG supplementation. Pediatrics. 2008. 121:e850–e856.36. Kalliomaki M, Salminen S, Poussa T, Isolauri E. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007. 119:1019–1021.37. Giovannini M, Agostoni C, Riva E, Salvini F, Ruscitto A, Zuccotti GV, Radaelli G. A randomized prospective double blind controlled trial on effects of long-term consumption of fermented milk containing Lactobacillus casei in pre-school children with allergic asthma and/or rhinitis. Pediatr Res. 2007. 62:215–220.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Asthma Prevention by Lactobacillus Rhamnosus in a Mouse Model is Associated With CD4+CD25+Foxp3+ T Cells
- Selection of Anti-Allergic Lactobacillus in Murine Model of Peanut Allergy
- The Effect of Lactobacillus acidophilus on the Primary Prevention of Asthma in a Murine Asthmatic Model
- The Immunologic Effects of Lactobacillus rhamnosus (Lcr35) Supplements in Adult Patients with Atopic Dermatitis
- Antimicrobial Effect of Lactobacillus in a Rat Model of Escherichia coli Urinary Tract Infection: A Preliminary Study