Allergy Asthma Immunol Res.
2012 May;4(3):150-156. 10.4168/aair.2012.4.3.150.
Asthma Prevention by Lactobacillus Rhamnosus in a Mouse Model is Associated With CD4+CD25+Foxp3+ T Cells
- Affiliations
-
- 1Asan Institute for Life Sciences, University of Ulsan College of Medicine, Seoul, Korea.
- 2Department of Pediatrics, Childhood Asthma Atopy Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sjhong@amc.seoul.kr
- 3Department of Pediatrics, Inje University Haeundae Paik Hospital, Busan, Korea.
- KMID: 2167067
- DOI: http://doi.org/10.4168/aair.2012.4.3.150
Abstract
- PURPOSE
Probiotic bacteria can induce immune regulation or immune tolerance in allergic diseases. The underlying mechanisms have been recently investigated, but are still unclear. The aim of this study was to evaluate the protective effects of the probiotic Lactobacillus rhamnosus (Lcr35) in a mouse model of asthma and to identify its mechanism of action.
METHODS
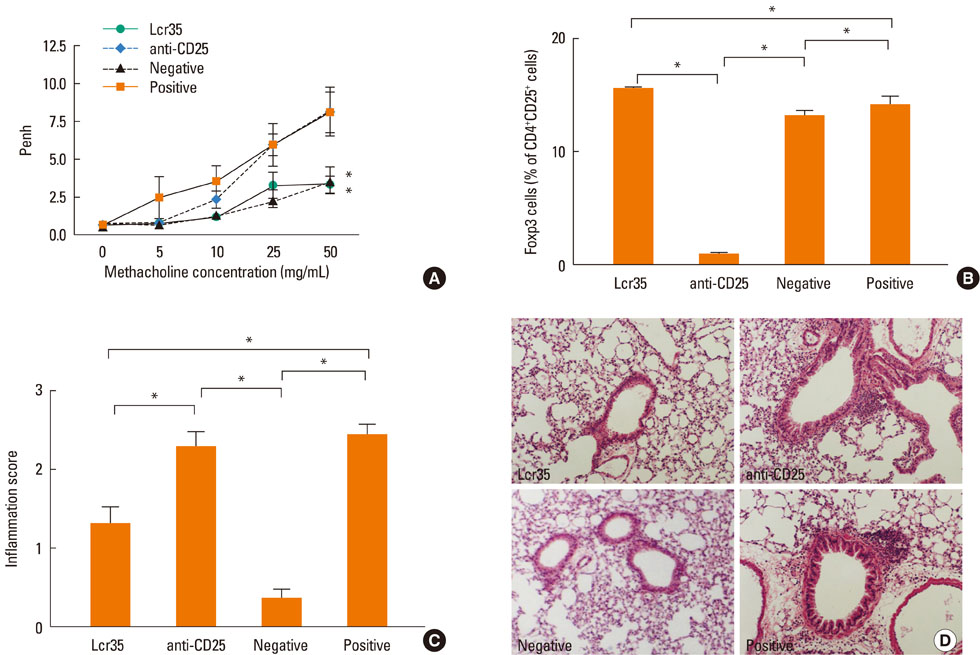
Lcr35 was administered daily by the oral route at a dosage of 1x10(9) CFU/mouse in BALB/c mice for 7 days before the first sensitization. Clinical parameters and regulatory T (Treg) cells were examined. The role of CD4+CD25+Foxp3+ Treg cells was analyzed using a Treg cell-depleting anti-CD25 monoclonal antibody (mAb).
RESULTS
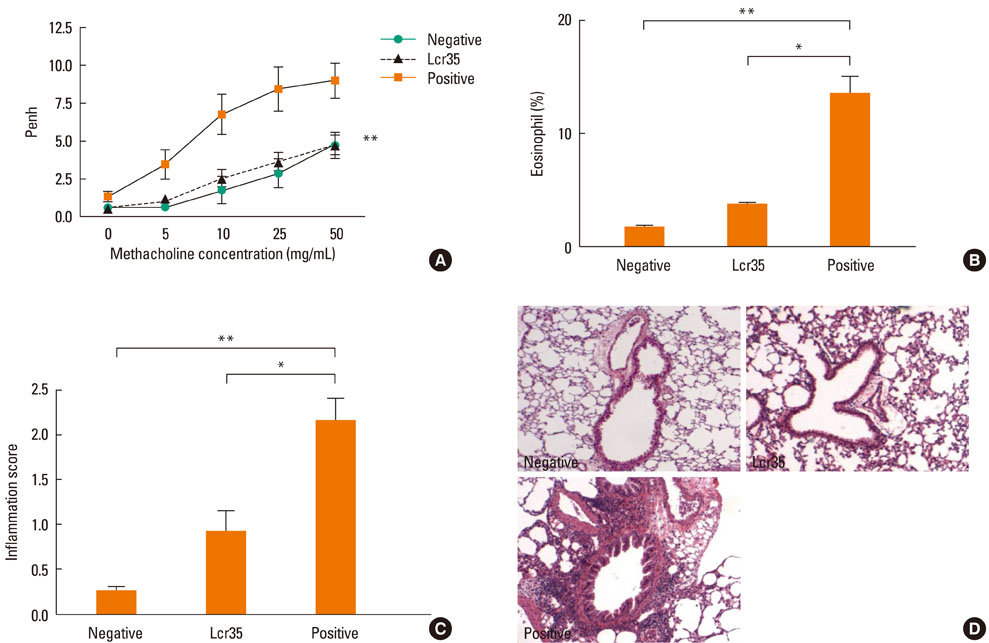
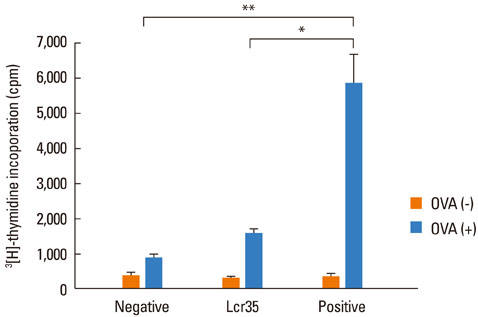
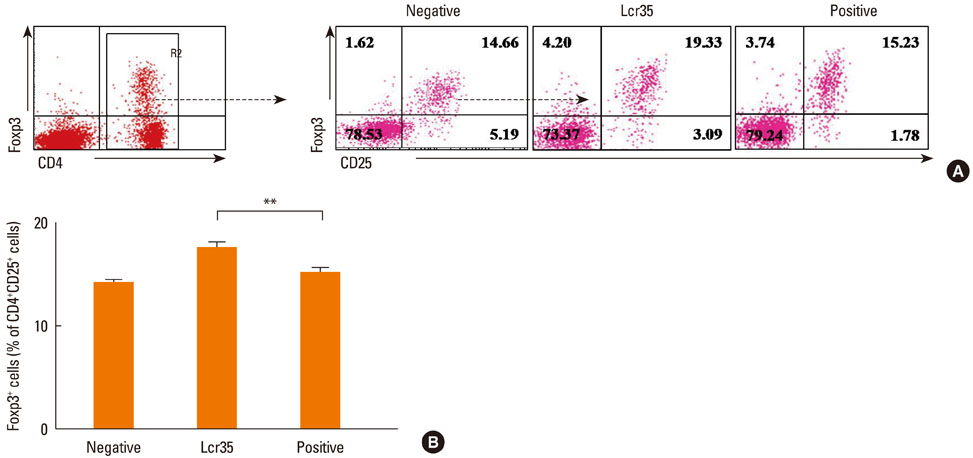
Airway hyperresponsiveness, total IgE production, pulmonary eosinophilic inflammation, and splenic lymphocyte proliferation were suppressed after Lcr35 treatment. Th1 (IFN-gamma) and Th2 (IL-4, IL-5, and IL-13) cytokines in the serum were suppressed, and the percentage of CD4+CD25+Foxp3+ Treg cells in the spleen was significantly increased in the Lcr35 treatment group. Anti-CD25 mAb administration abolished the protective effects of Lcr35, indicating that CD4+ CD25+Foxp3+ Treg cells are essential in mediating the activity of Lcr35.
CONCLUSIONS
Oral administration of Lcr35 attenuated the features of allergic asthma in a mouse model and induced immune regulation by a CD4+CD25+Foxp3+ Treg cell-mediated mechanism.
Keyword
MeSH Terms
Figure
Reference
-
1. Marteau P. Probiotics, prebiotics, synbiotics: ecological treatment for inflammatory bowel disease? Gut. 2006. 55:1692–1693.2. Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, Nam JH, Rhee JH, Hwang KC, Im SH. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci U S A. 2010. 107:2159–2164.3. Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004. 303:1662–1665.4. Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000. 191:435–444.5. Feleszko W, Jaworska J, Rha RD, Steinhausen S, Avagyan A, Jaudszus A, Ahrens B, Groneberg DA, Wahn U, Hamelmann E. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 2007. 37:498–505.6. Hong HJ, Kim E, Cho D, Kim TS. Differential suppression of heat-killed lactobacilli isolated from kimchi, a Korean traditional food, on airway hyper-responsiveness in mice. J Clin Immunol. 2010. 30:449–458.7. Yu J, Jang SO, Kim BJ, Song YH, Kwon JW, Kang MJ, Choi WA, Jung HD, Hong SJ. The effects of Lactobacillus rhamnosus on the prevention of asthma in a murine model. Allergy Asthma Immunol Res. 2010. 2:199–205.8. Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001. 357:1076–1079.9. Helin T, Haahtela S, Haahtela T. No effect of oral treatment with an intestinal bacterial strain, Lactobacillus rhamnosus (ATCC 53103), on birch-pollen allergy: a placebo-controlled double-blind study. Allergy. 2002. 57:243–246.10. Shida K, Takahashi R, Iwadate E, Takamizawa K, Yasui H, Sato T, Habu S, Hachimura S, Kaminogawa S. Lactobacillus casei strain Shirota suppresses serum immunoglobulin E and immunoglobulin G1 responses and systemic anaphylaxis in a food allergy model. Clin Exp Allergy. 2002. 32:563–570.11. Pochard P, Gosset P, Grangette C, Andre C, Tonnel AB, Pestel J, Mercenier A. Lactic acid bacteria inhibit Th2 cytokine production by mononuclear cells from allergic patients. J Allergy Clin Immunol. 2002. 110:617–623.12. Smits HH, Engering A, van der Kleij D, de Jong EC, Schipper K, van Capel TM, Zaat BA, Yazdanbakhsh M, Wierenga EA, van Kooyk Y, Kapsenberg ML. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005. 115:1260–1267.13. Tournoy KG, Kips JC, Schou C, Pauwels RA. Airway eosinophilia is not a requirement for allergen-induced airway hyperresponsiveness. Clin Exp Allergy. 2000. 30:79–85.14. Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME. Probiotics and immunity. J Gastroenterol. 2009. 44:26–46.15. Torii A, Torii S, Fujiwara S, Tanaka H, Inagaki N, Nagai H. Lactobacillus acidophilus strain L-92 regulates the production of Th1 cytokine as well as Th2 cytokines. Allergol Int. 2007. 56:293–301.16. Segawa S, Nakakita Y, Takata Y, Wakita Y, Kaneko T, Kaneda H, Watari J, Yasui H. Effect of oral administration of heat-killed Lactobacillus brevis SBC8803 on total and ovalbumin-specific immunoglobulin E production through the improvement of Th1/Th2 balance. Int J Food Microbiol. 2008. 121:1–10.17. Mastrangeli G, Corinti S, Butteroni C, Afferni C, Bonura A, Boirivant M, Colombo P, Di Felice G. Effects of live and inactivated VSL#3 probiotic preparations in the modulation of in vitro and in vivo allergen-induced Th2 responses. Int Arch Allergy Immunol. 2009. 150:133–143.18. Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005. 202:1539–1547.19. Kearley J, Robinson DS, Lloyd CM. CD4+CD25+ regulatory T cells reverse established allergic airway inflammation and prevent airway remodeling. J Allergy Clin Immunol. 2008. 122:617–624.e6.20. McHugh RS, Shevach EM. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J Immunol. 2002. 168:5979–5983.21. Kohm AP, Williams JS, Miller SD. Cutting edge: ligation of the glucocorticoid-induced TNF receptor enhances autoreactive CD4+ T cell activation and experimental autoimmune encephalomyelitis. J Immunol. 2004. 172:4686–4690.22. Kohm AP, Williams JS, Bickford AL, McMahon JS, Chatenoud L, Bach JF, Bluestone JA, Miller SD. Treatment with nonmitogenic anti-CD3 monoclonal antibody induces CD4+ T cell unresponsiveness and functional reversal of established experimental autoimmune encephalomyelitis. J Immunol. 2005. 174:4525–4534.23. Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz DJ, Ziegler SF, Miller SD. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol. 2006. 176:3301–3305.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on the Number of Circulating CD4+CD25+Foxp3+ Regulatory T Cells and CD4+CD25-Foxp3+ T Cells in Psoriasis
- Peripheral Generation of CD4+ CD25+ Foxp3+ Regulatory T Cells
- Dynamic Frequency of Blood CD4+CD25+ Regulatory T Cells in Rats with Collagen-induced Arthritis
- Alteration of CD4+CD25+Foxp3+ T cell level in Kawasaki disease
- A Novel Synthetic Mycolic Acid Inhibits Bronchial Hyperresponsiveness and Allergic Inflammation in a Mouse Model of Asthma