Korean J Urol.
2006 Apr;47(4):418-425. 10.4111/kju.2006.47.4.418.
The Inhibition of Human Telomerase Reverse Transcriptase Expression by Peroxiredoxin I and c-Myc in Prostatic Cancer Cells
- Affiliations
-
- 1Department of Urology, Seoul National University College of Medicine, Seoul, Korea.
- 2Division of Brain Disease, Department of Biochemical Sciences, National Institute of Health Korea Center for Disease Control and Prevention, Seoul, Korea.
- 3Department of Urology, College of Medicine, Chung-Ang University, Seoul, Korea.
- KMID: 1997154
- DOI: http://doi.org/10.4111/kju.2006.47.4.418
Abstract
- PURPOSE
We evaluated the hypothesis that the telomerase expression is associated with c-Myc and peroxiredoxin I (Prx I) in patients with prostate cancer. The study determined the link between Prx I, c-Myc and human telomerase reverse transcriptase (hTERT) in prostate cancer cells.
MATERIALS AND METHODS
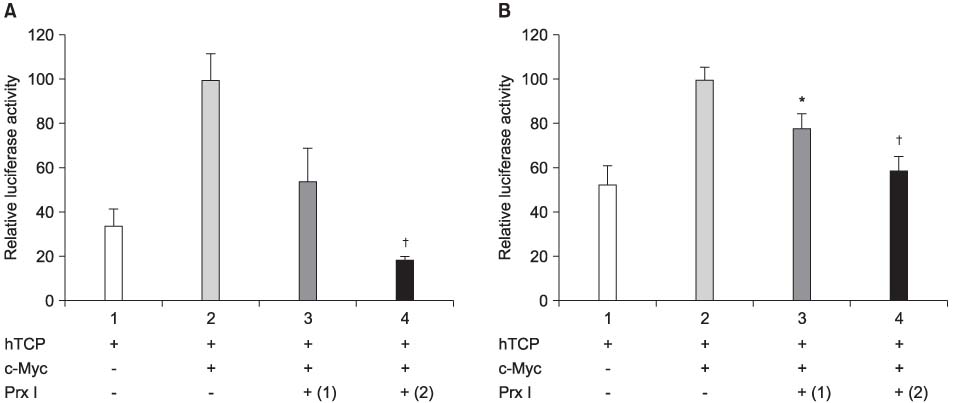
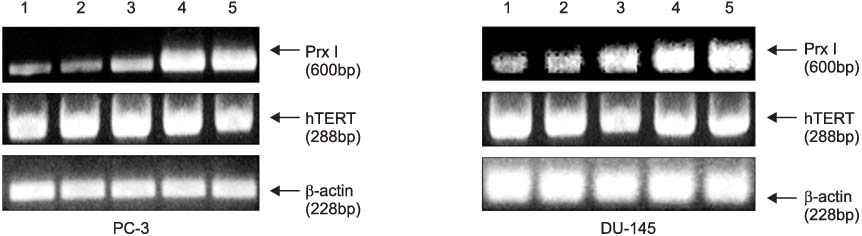
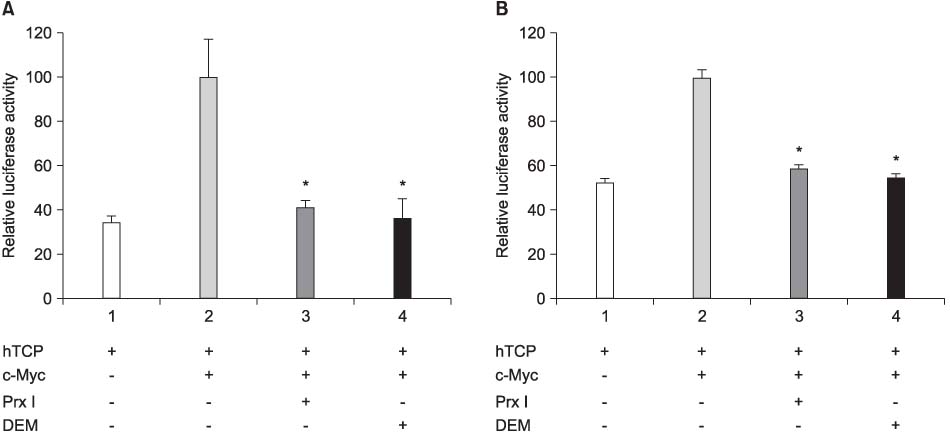
The cDNA of the Prx I gene was obtained by reverse-transcriptase polymerase chain reaction (RT-PCR) amplification. Cotransfections were performed by using a hTERT luciferase reporter plasmid and each expression vector as indicated (c-Myc or Prx I). Empty vectors were used as controls for determining the basal promoter activity. RT-PCR was performed to evaluate the effect of the DEM-induced Prx I mRNA expression. Luciferase assay was performed to evaluate the inhibitory effect of transfected Prx I and the DEM induced Prx I on the transcriptional activity of hTERT in the human prostatic cancer cell lines PC-3 and DU-145.
RESULTS
In this study, we found that Prx I could inhibit hTERT expression through direct interaction with c-Myc protein in the prostate cancer cell lines. In addition, it was obvious that Prx I could interact with c-Myc protein. We also found that DEM could induce upregulation of the Prx I mRNA expression and that the increased expression of Prx I could downregulate the expression of hTERT.
CONCLUSIONS
Our results demonstrated a direct link between Prx I, c-Myc and hTERT, and we suggest that Prx I regulates cellular immortalization through c-Myc and hTERT, which is activation step in carcinogenesis.
MeSH Terms
Figure
Reference
-
1. Lee C, Lee ES, Choi H, Koh SK, Lee JM, Chai SE, et al. Incidence estimation of genitourinary cancer in Korea. J Korean Med Sci. 1992. 7:154–161.2. Schroder FH. Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Hormonal therapy of prostate cancer. Campbell's urology. 2002. 8th ed. Philadelphia: Saunders;3182–3208.3. Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998. 279:349–352.4. Blackburn EH. Structure and function of telomeres. Nature. 1991. 350:569–573.5. Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985. 43:405–413.6. Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, et al. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992. 11:1921–1929.7. Prendergast GC, Lawe D, Ziff EB. Association of Myn, the murine homolog of max, with c-Myc stimulates methylation-sensitive DNA binding and ras cotransformation. Cell. 1991. 65:395–407.8. McMahon SB, Wood MA, Cole MD. The essential cofactor TRRAP recruits the histone acetyltransferase hGCN5 to c-Myc. Mol Cell Biol. 2000. 20:556–562.9. Kyo S, Takakura M, Taira T, Kanaya T, Itoh H, Yutsudo M, et al. Sp1 cooperates with c-Myc to activate transcription of the human telomerase reverse transcriptase gene (hTERT). Nucleic Acids Res. 2000. 28:669–677.10. Kinnula VL, Paakko P, Soini Y. Antioxidant enzymes and redox regulating thiol proteins in malignancies of human lung. FEBS Lett. 2004. 569:1–6.11. Rhee SG, Kang SW, Chang TS, Jeong W, Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001. 52:35–41.12. Prosperi MT, Ferbus D, Karczinski I, Goubin G. A human cDNA corresponding to a gene overexpressed during cell proliferation encodes a product sharing homology with amoebic and bacterial proteins. J Biol Chem. 1993. 268:11050–11056.13. Prosperi MT, Ferbus D, Rouillard D, Goubin G. The pag gene product, a physiological inhibitor of c-abl tyrosine kinase, is overexpressed in cells entering S phase and by contact with agents inducing oxidative stress. FEBS Letters. 1998. 423:39–44.14. Mu ZM, Yin XY, Prochownik EV. Pag, a putative tumor suppressor, interacts with the Myc Box II domain of c-Myc and selectively alters its biological function and target gene expression. J Biol Chem. 2002. 277:43175–43184.15. Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000. 2000:PE1.16. Wang Z, Kyo S, Takakura M, Tanaka M, Yatabe N, Maida Y, et al. Progesterone regulates human telomerase reverse transcriptase gene expression via activation of mitogen-activated protein kinase signaling pathway. Cancer Res. 2000. 60:5376–5381.17. Lin Y, Uemura H, Fujinami K, Hosaka M, Harada M, Kubotay Y. Telomerase activity in primary prostate cancer. J Urol. 1997. 157:1161–1165.18. Shen C, Nathan C. Nonredundant antioxidant defense by multiple two-cysteine peroxiredoxins in human prostate cancer cells. Mol Med. 2002. 8:95–102.19. Nag A, Smith RG. Amplification, rearrangement and elevated expression of c-myc in the human prostatic-carcinoma cell line LNCaP. Prostate. 1989. 15:115–122.20. Latil A, Vidaud D, Valeri A, Fournier G, Vidaud M, Lidereau R, et al. Htert expression correlates with MYC over-expression in human prostate cancer. Int J Cancer. 2000. 89:172–176.21. Zhou C, Liu J. Inhibition of human telomerase reverse transcriptase gene expression by BRCA1 in human ovarian cancer cells. Biochem Biophys Res Commun. 2003. 303:130–136.22. Li H, Lee TH, Avraham H. A novel tricomplex of BRCA1, Nmi, and c-Myc inhibits c-Myc-induced human telomerase reverse transcriptase gene (hTERT) promoter activity in breast cancer. J Biol Chem. 2002. 277:20965–20973.23. Bernard D, Pourtier-Manzanedo A, Gil J, Beach DH. Myc confers androgen-independent prostate cancer cell growth. J Clin Invest. 2003. 112:1724–1731.24. Biroccio A, Leonetti C. Telomerase as a new target for the treatment of hormone-refractory prostate cancer. Endocrine-Related Cancer. 2004. 11:407–421.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of Telomerase Activity and Proliferation by Inhibition of Reverse Transcriptase Activity in Human Cancer Cell
- Telomerase Activity and Expression of hTR and TERT in Human Soft Tissue Sarcomas
- Protein kinase C modulates telomerase activity in human cervical cancer cells
- Effect on Cell Growth, c-myc mRNA Expression and Telomerase Activity by Transforming Growth Factor-beta1 in Malignant Lymphoma and Leukemia Cell Line
- Change of the Expression of Human Telomerase Reverse Transcriptase mRNA and Human Telomerase RNA after Cisplatin and 5-Fluorouracil Exposure in Head and Neck Squamous Cell Carcinoma Cell Lines