Allergy Asthma Immunol Res.
2012 Jul;4(4):206-213. 10.4168/aair.2012.4.4.206.
Asian Sand Dust Enhances Allergen-Induced Th2 Allergic Inflammatory Changes and Mucin Production in BALB/c Mouse Lungs
- Affiliations
-
- 1Department of Otorhinolaryngology, Gil Hospital, Graduate School of Medicine, Gachon University of Medicine and Science, Incheon, Korea. rhinokim2002@hanmail.net
- KMID: 1970709
- DOI: http://doi.org/10.4168/aair.2012.4.4.206
Abstract
- PURPOSE
Recent studies have reported that Asian sand dust (ASD) has a potential risk of aggravating airway inflammation. The purpose of this study was to investigate the effect of ASD on inflammation and mucin production in the airways of allergic mice.
METHODS
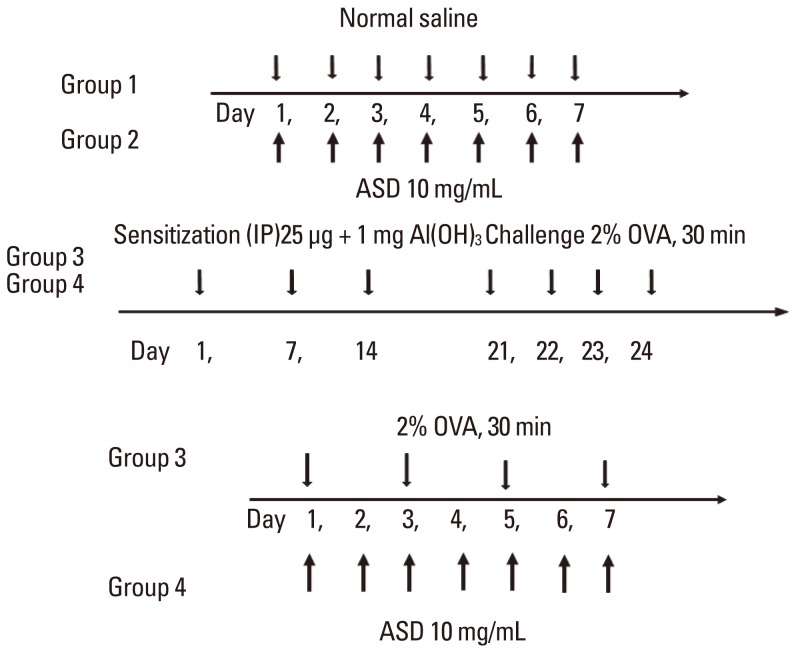
Forty BALB/c female mice were divided into four groups: saline (group 1); ASD (group 2); ovalbumin (OVA) alone (group 3); and OVA+ASD (group 4). OVA-specific immunoglobulin E (IgE) in serum and interleukin (IL)-4, IL-5, IL-13, and interferon-gamma (IFN-gamma) in bronchoalveolar lavage fluid (BALF) were measured by enzyme-linked immunosorbent assay (ELISA). Hematoxylin & eosin (H&E) and Periodic acid-Schiff (PAS) staining was performed on lung tissues. In addition, immunohistochemical staining for IL-4, IL-5, MUC5AC, and transforming growth factor alpha (TGF-alpha) was conducted.
RESULTS
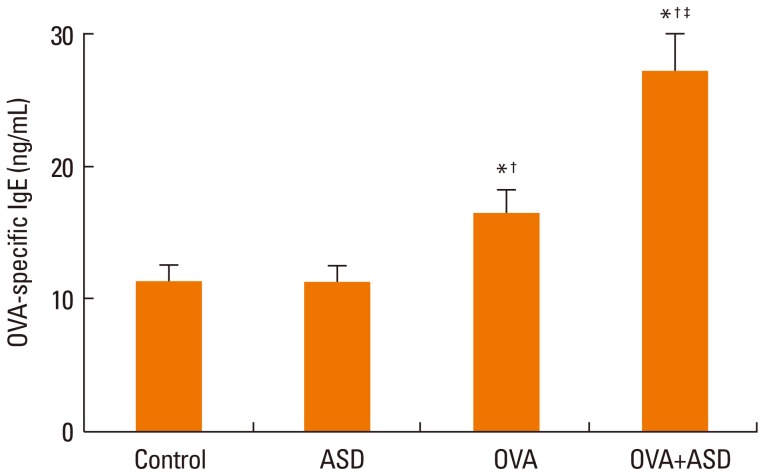
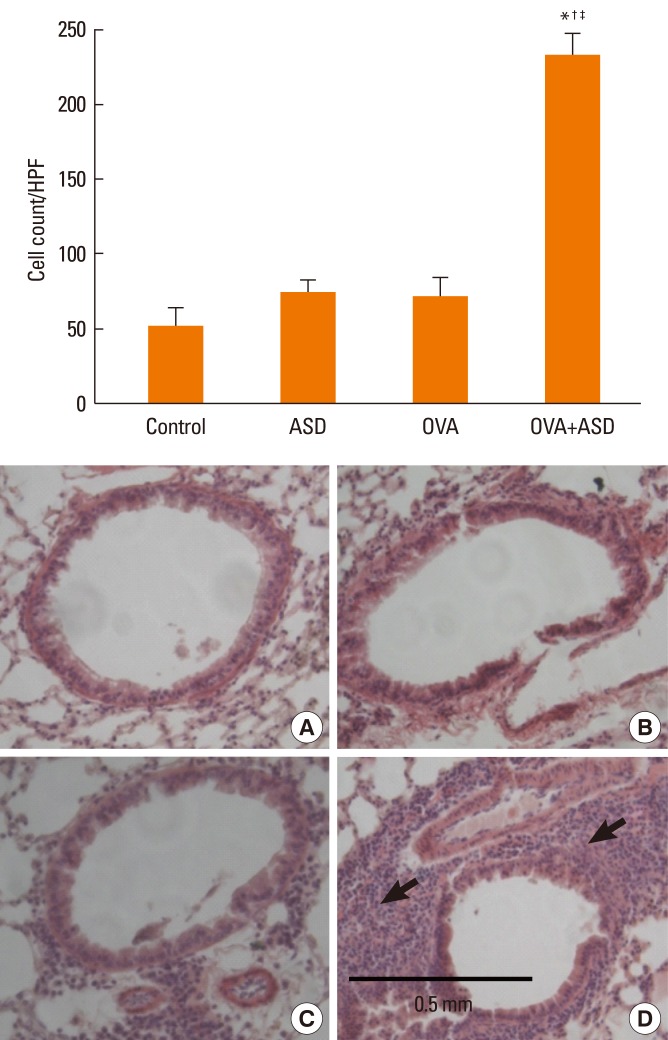
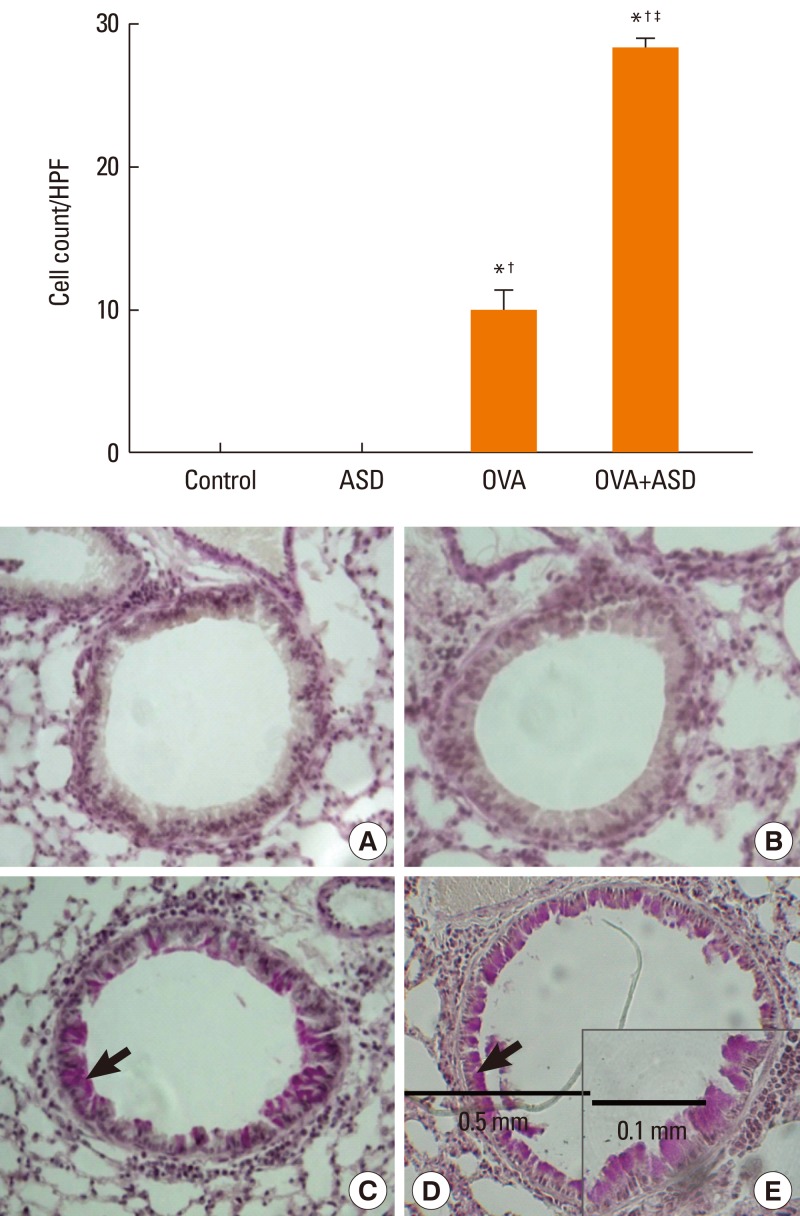
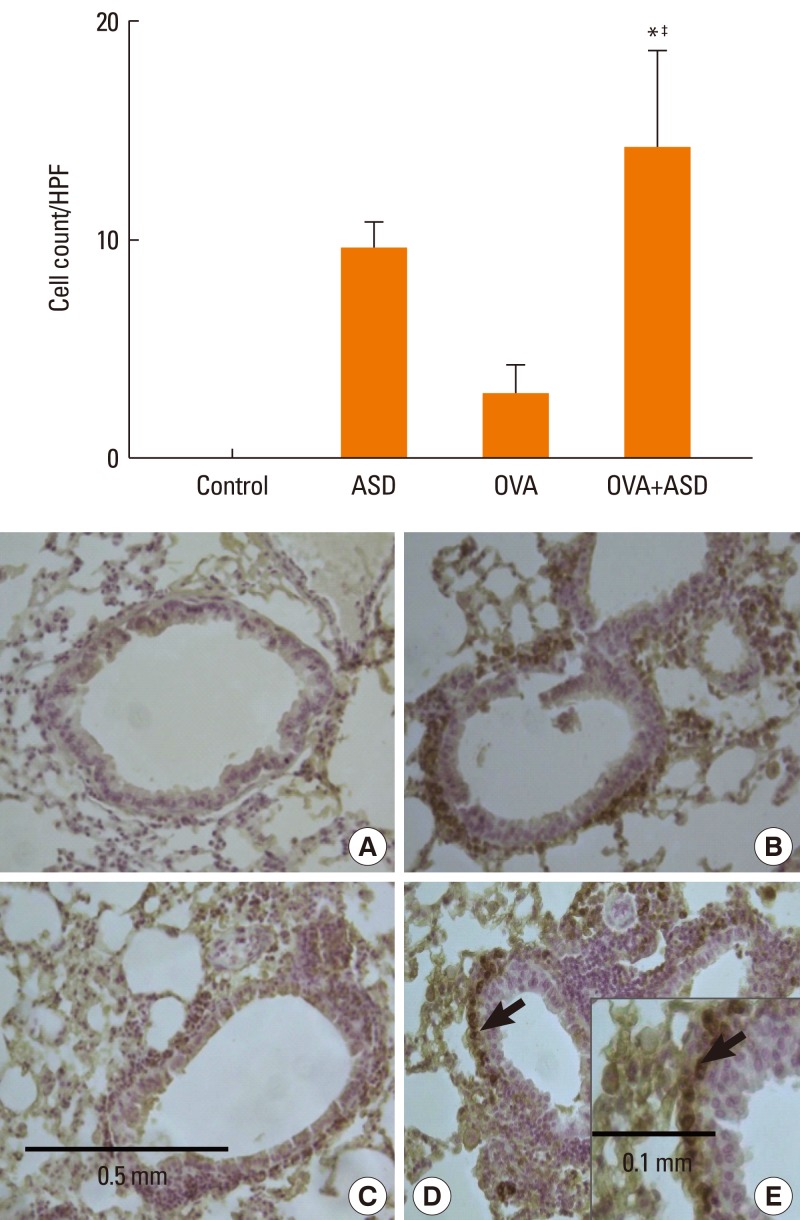
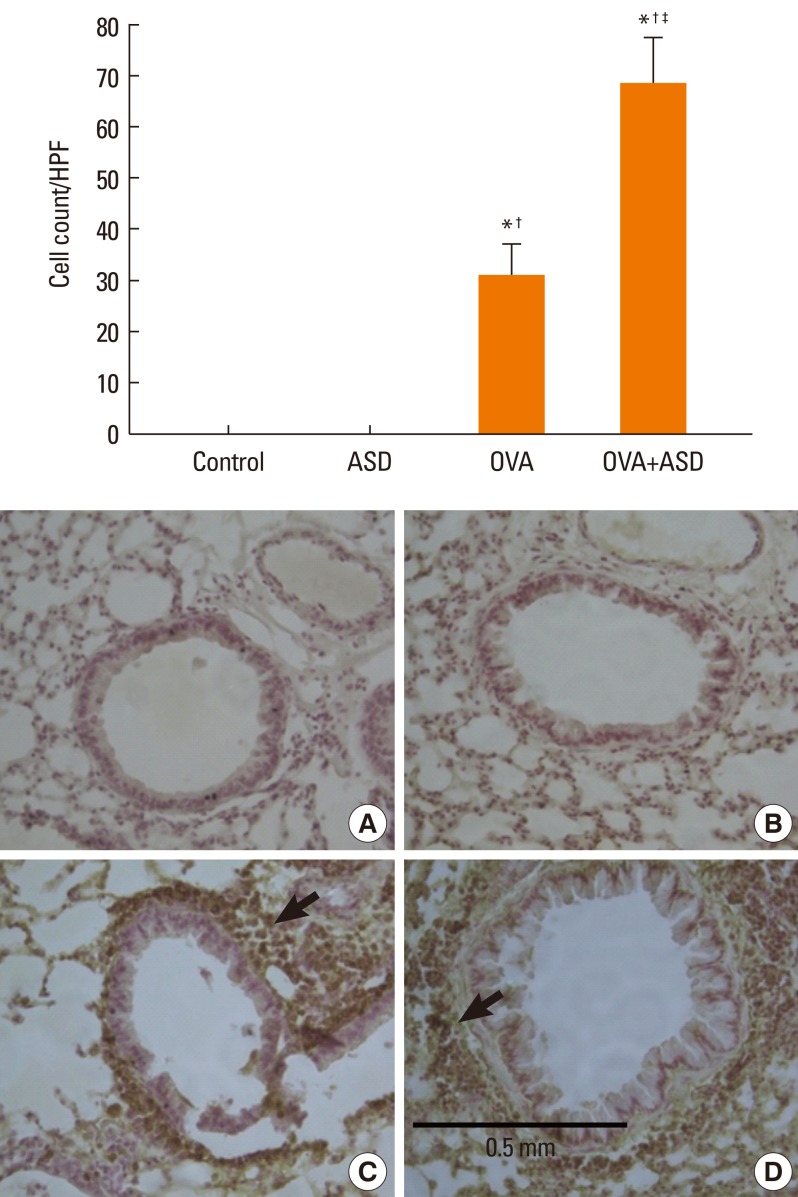
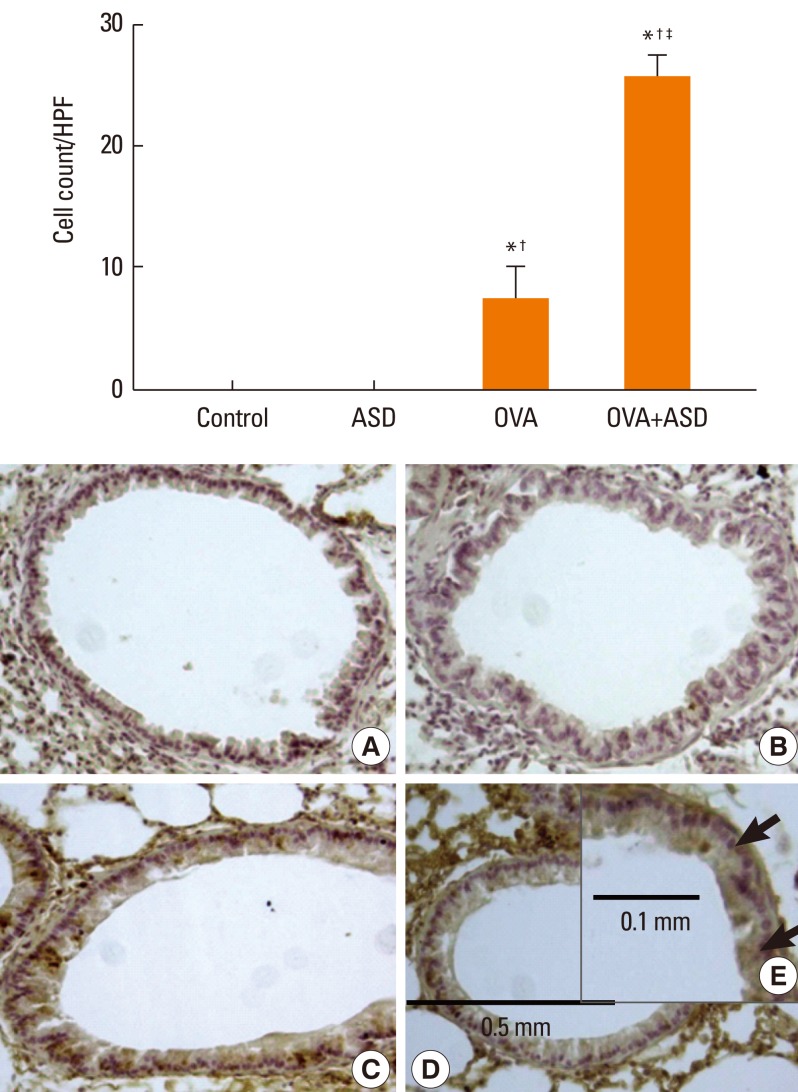
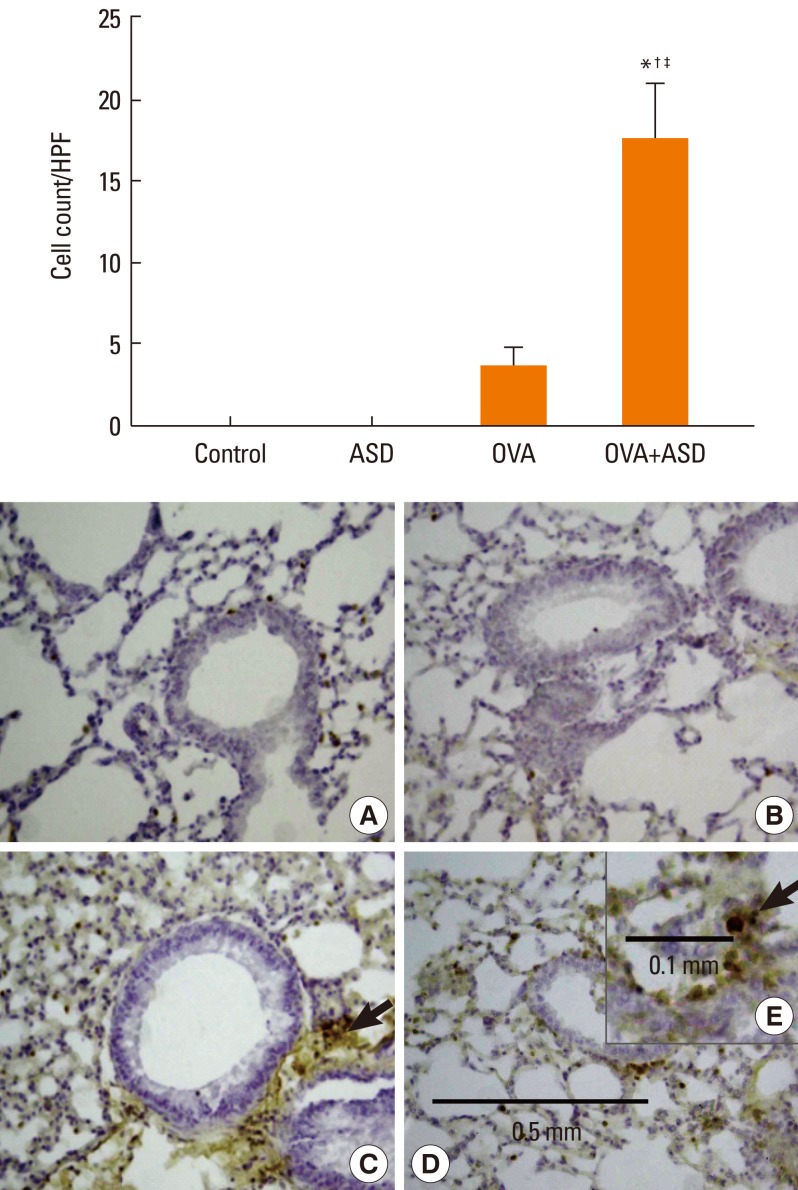
Serum IgE levels were significantly higher in group 4 than in group 3 (P<0.05). IL-4 and IL-5 in BALF were significantly higher in group 4 than in group 3 (P<0.05, respectively). Based on H&E staining, inflammatory cell numbers were significantly greater in group 4 than in the other groups (P<0.05). The number of PAS-positive cells was also significantly greater in groups 3 and 4 than in groups 1 and 2 (P<0.05). The numbers of IL-4 and IL-5-positive cells were higher in group 4 than in group 3 (P<0.05). The number of MUC5AC and TGF-alpha-positive cells were also higher in group 4 than in group 3 (P<0.05).
CONCLUSIONS
Our data suggest that ASD increases cytokine expression and mucin production in an allergic murine model. The increased inflammatory reactions were related to cytokine production.
Keyword
MeSH Terms
-
Animals
Asian Continental Ancestry Group
Bronchoalveolar Lavage Fluid
Cell Count
Dust
Enzyme-Linked Immunosorbent Assay
Eosine Yellowish-(YS)
Female
Hematoxylin
Humans
Immunoglobulin E
Immunoglobulins
Inflammation
Interferon-gamma
Interleukin-13
Interleukin-4
Interleukin-5
Interleukins
Lung
Mice
Mucins
Ovalbumin
Silicon Dioxide
Transforming Growth Factor alpha
Dust
Eosine Yellowish-(YS)
Hematoxylin
Immunoglobulin E
Immunoglobulins
Interferon-gamma
Interleukin-13
Interleukin-4
Interleukin-5
Interleukins
Mucins
Ovalbumin
Silicon Dioxide
Transforming Growth Factor alpha
Figure
Cited by 2 articles
-
Asian Sand Dust Enhances the Inflammatory Response and Mucin Gene Expression in the Middle Ear
Jiwon Chang, Yoon Young Go, Moo Kyun Park, Sung-Won Chae, Seon-Heui Lee, Jae-Jun Song
Clin Exp Otorhinolaryngol. 2016;9(3):198-205. doi: 10.21053/ceo.2015.01060.Microarray Analysis of Gene Expression Alteration in Human Middle Ear Epithelial Cells Induced by Asian Sand Dust
Yoon Young Go, Moo Kyun Park, Jee Young Kwon, Young Rok Seo, Sung-Won Chae, Jae-Jun Song
Clin Exp Otorhinolaryngol. 2015;8(4):345-353. doi: 10.3342/ceo.2015.8.4.345.
Reference
-
1. Salvi S. Pollution and allergic airways disease. Curr Opin Allergy Clin Immunol. 2001; 1:35–41. PMID: 11964667.
Article2. Kim YK, Song SK, Lee HW, Kim CH, Oh IB, Moon YS, Shon ZH. Characteristics of Asian dust transport based on synoptic meteorological analysis over Korea. J Air Waste Manag Assoc. 2006; 56:306–316. PMID: 16573193.
Article3. Kang J, Choi MS, Lee CB. Atmospheric metal and phosphorus concentrations, inputs, and their biogeochemical significances in the Japan/East Sea. Sci Total Environ. 2009; 407:2270–2284. PMID: 19135230.
Article4. Duce RA, Unni CK, Ray BJ, Prospero JM, Merrill JT. Long-range atmospheric transport of soil dust from Asia to the tropical north pacific: temporal variability. Science. 1980; 209:1522–1524. PMID: 17745962.
Article5. Park SH, Song CB, Kim MC, Kwon SB, Lee KW. Study on size distribution of total aerosol and water-soluble ions during an Asian dust storm event at Jeju Island, Korea. Environ Monit Assess. 2004; 93:157–183. PMID: 15074615.
Article6. Han JS, Moon KJ, Ahn JY, Hong YD, Kim YJ, Ryu SY, Cliff SS, Cahill TA. Characteristics of ion components and trace elements of fine particles at Gosan, Korea in spring time from 2001 to 2002. Environ Monit Assess. 2004; 92:73–93. PMID: 15038537.
Article7. Park JW, Lim YH, Kyung SY, An CH, Lee SP, Jeong SH, Ju YS. Effects of ambient particulate matter on peak expiratory flow rates and respiratory symptoms of asthmatics during Asian dust periods in Korea. Respirology. 2005; 10:470–476. PMID: 16135170.
Article8. Hong YC, Pan XC, Kim SY, Park K, Park EJ, Jin X, Yi SM, Kim YH, Park CH, Song S, Kim H. Asian Dust Storm and pulmonary function of school children in Seoul. Sci Total Environ. 2010; 408:754–759. PMID: 19939437.
Article9. Kwon HJ, Cho SH, Chun Y, Lagarde F, Pershagen G. Effects of the Asian dust events on daily mortality in Seoul, Korea. Environ Res. 2002; 90:1–5. PMID: 12359184.
Article10. Lee JT, Son JY, Cho YS. A comparison of mortality related to urban air particles between periods with Asian dust days and without Asian dust days in Seoul, Korea, 2000-2004. Environ Res. 2007; 105:409–413. PMID: 17659273.
Article11. Lei YC, Chan CC, Wang PY, Lee CT, Cheng TJ. Effects of Asian dust event particles on inflammation markers in peripheral blood and bronchoalveolar lavage in pulmonary hypertensive rats. Environ Res. 2004; 95:71–76. PMID: 15068932.
Article12. Ichinose T, Sadakane K, Takano H, Yanagisawa R, Nishikawa M, Mori I, Kawazato H, Yasuda A, Hiyoshi K, Shibamoto T. Enhancement of mite allergen-induced eosinophil infiltration in the murine airway and local cytokine/chemokine expression by Asian sand dust. J Toxicol Environ Health A. 2006; 69:1571–1585. PMID: 16854786.
Article13. Hiyoshi K, Ichinose T, Sadakane K, Takano H, Nishikawa M, Mori I, Yanagisawa R, Yoshida S, Kumagai Y, Tomura S, Shibamoto T. Asian sand dust enhances ovalbumin-induced eosinophil recruitment in the alveoli and airway of mice. Environ Res. 2005; 99:361–368. PMID: 16307978.
Article14. He M, Ichinose T, Yoshida S, Nishikawa M, Mori I, Yanagisawa R, Takano H, Inoue K, Sun G, Shibamoto T. Airborne Asian sand dust enhances murine lung eosinophilia. Inhal Toxicol. 2010; 22:1012–1025. PMID: 20849355.
Article15. Yeo NK, Hwang YJ, Kim ST, Kwon HJ, Jang YJ. Asian sand dust enhances rhinovirus-induced cytokine secretion and viral replication in human nasal epithelial cells. Inhal Toxicol. 2010; 22:1038–1045. PMID: 20879958.
Article16. Pène J, Rousset F, Brière F, Chrétien I, Bonnefoy JY, Spits H, Yokota T, Arai N, Arai K, Banchereau J, et al. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proc Natl Acad Sci U S A. 1988; 85:6880–6884. PMID: 2970644.17. Ichinose T, Yoshida S, Sadakane K, Takano H, Yanagisawa R, Inoue K, Nishikawa M, Mori I, Kawazato H, Yasuda A, Shibamoto T. Effects of asian sand dust, Arizona sand dust, amorphous silica and aluminum oxide on allergic inflammation in the murine lung. Inhal Toxicol. 2008; 20:685–694. PMID: 18464056.
Article18. Donaldson K, Stone V, Clouter A, Renwick L, MacNee W. Ultrafine particles. Occup Environ Med. 2001; 58:211–216. PMID: 11171936.19. Kim KH, Kim MY. The effects of Asian Dust on particulate matter fractionation in Seoul, Korea during spring 2001. Chemosphere. 2003; 51:707–721. PMID: 12668030.
Article20. Ichinose T, Hiyoshi K, Yoshida S, Takano H, Inoue K, Nishikawa M, Mori I, Kawazato H, Yasuda A, Shibamoto T. Asian sand dust aggravates allergic rhinitis in guinea pigs induced by Japanese cedar pollen. Inhal Toxicol. 2009; 21:985–993. PMID: 19552583.21. Ali MS, Wilson JA, Bennett M, Pearson JP. Mucin gene expression in nasal polyps. Acta Otolaryngol. 2005; 125:618–624. PMID: 16076710.
Article22. Dabbagh K, Takeyama K, Lee HM, Ueki IF, Lausier JA, Nadel JA. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol. 1999; 162:6233–6237. PMID: 10229869.23. Louahed J, Toda M, Jen J, Hamid Q, Renauld JC, Levitt RC, Nicolaides NC. Interleukin-9 upregulates mucus expression in the airways. Am J Respir Cell Mol Biol. 2000; 22:649–656. PMID: 10837360.
Article24. Nadel JA. Mechanisms of airway hypersecretion and novel therapy. Chest. 2000; 117:262S–266S. PMID: 10843942.
Article25. Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF, Nadel JA. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol. 2000; 164:1546–1552. PMID: 10640773.
Article26. Hwang YJ, Jeung YS, Seo MH, Yoon JY, Kim DY, Park JW, Han JH, Jeong SH. Asian dust and titanium dioxide particles-induced inflammation and oxidative DNA damage in C57BL/6 mice. Inhal Toxicol. 2010; 22:1127–1133. PMID: 21070184.
Article27. Naota M, Mukaiyama T, Shimada A, Yoshida A, Okajima M, Morita T, Inoue K, Takano H. Pathological study of acute pulmonary toxicity induced by intratracheally instilled Asian sand dust (kosa). Toxicol Pathol. 2010; 38:1099–1110. PMID: 20884819.
Article28. Coles SJ, Levine LR, Reid L. Hypersecretion of mucus glycoproteins in rat airways induced by tobacco smoke. Am J Pathol. 1979; 94:459–472. PMID: 426035.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Asian Sand Dust in Allergic Inflammation of Allergic Mouse
- 4-CMTB Ameliorates Ovalbumin-Induced Allergic Asthma through FFA2 Activation in Mice
- Understanding the Mouse Model of Respiratory Allergic Diseases
- Anti-Allergic Effect of Oroxylin A from Oroxylum indicum Using in vivo and in vitro Experiments
- The Effect of Interleukin-12 on the Immune Response in Allergic Rhinitis Mouse Model