J Korean Rheum Assoc.
2007 Jun;14(2):125-135. 10.4078/jkra.2007.14.2.125.
Expression and Function of Plexin A1 in Rheumatoid Synoviocytes
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine,The Catholic University of Korea College of Medicine, Seoul, Korea. wan725@catholic.ac.kr
- KMID: 1526429
- DOI: http://doi.org/10.4078/jkra.2007.14.2.125
Abstract
Objective
To investigate the expression and function of plexin A1, a transmembrane protein involving cell survival and cell-to cell interaction, in the rheumatoid synoviocytes.
Methods
Immunohistochemical staining using anti-plexin A1 antibody was performed in the synovium of rheumatoid arthritis (RA) patients. The plexin A1 expression in cultured fibroblast-like synovioytes (FLS) was also examined by Western blot analysis and immunocytochemistry. Cell viability was determined by CCK-8 assay. Deficiency of plexin A1 was established by the method of short interfering RNA (siRNA). The productions of interleukin-6 (IL-6) and monocytes chemotactic protein-1 (MCP-1) were measured in culture supernatants by ELISA.
Results
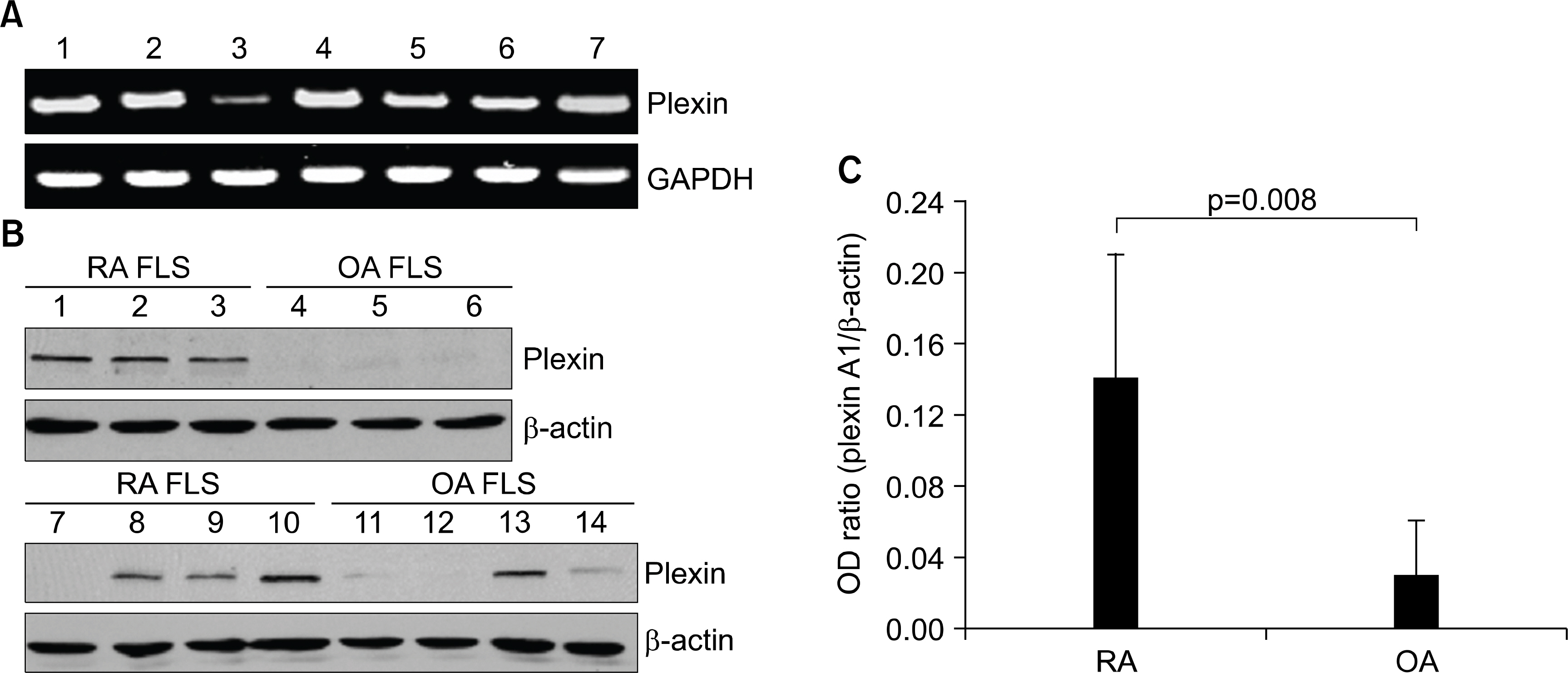
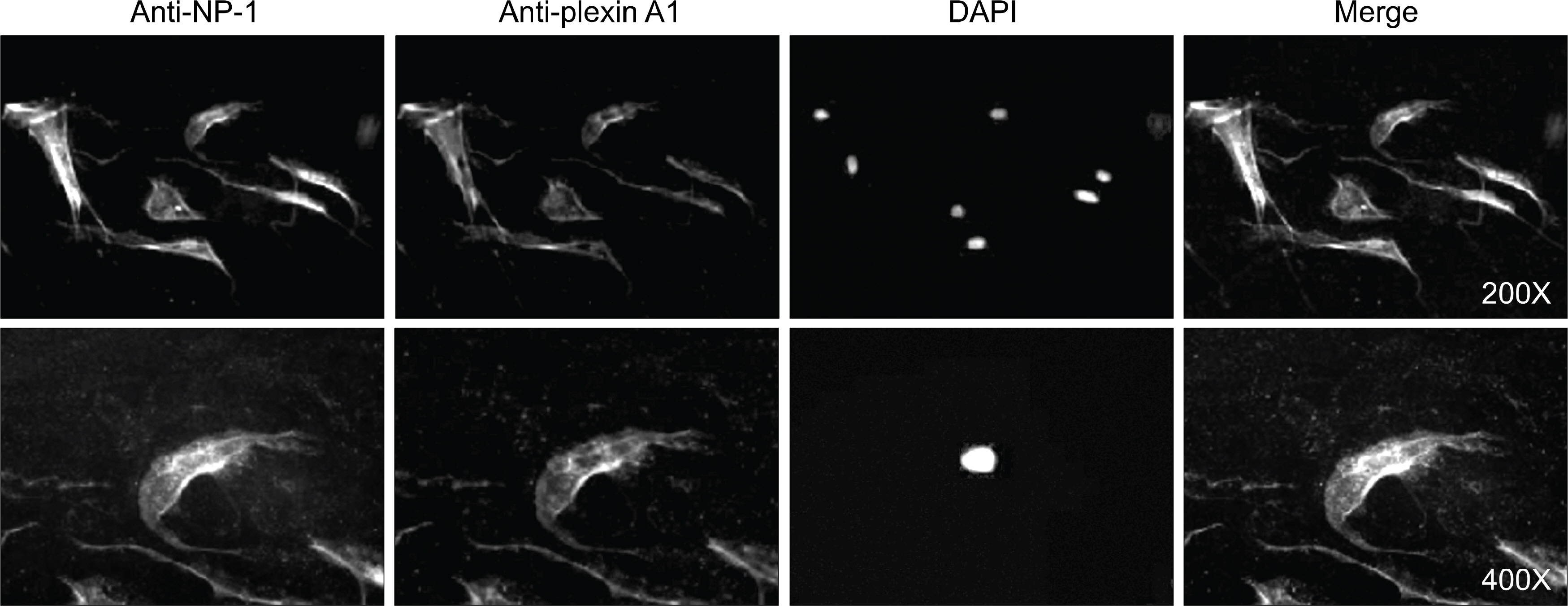
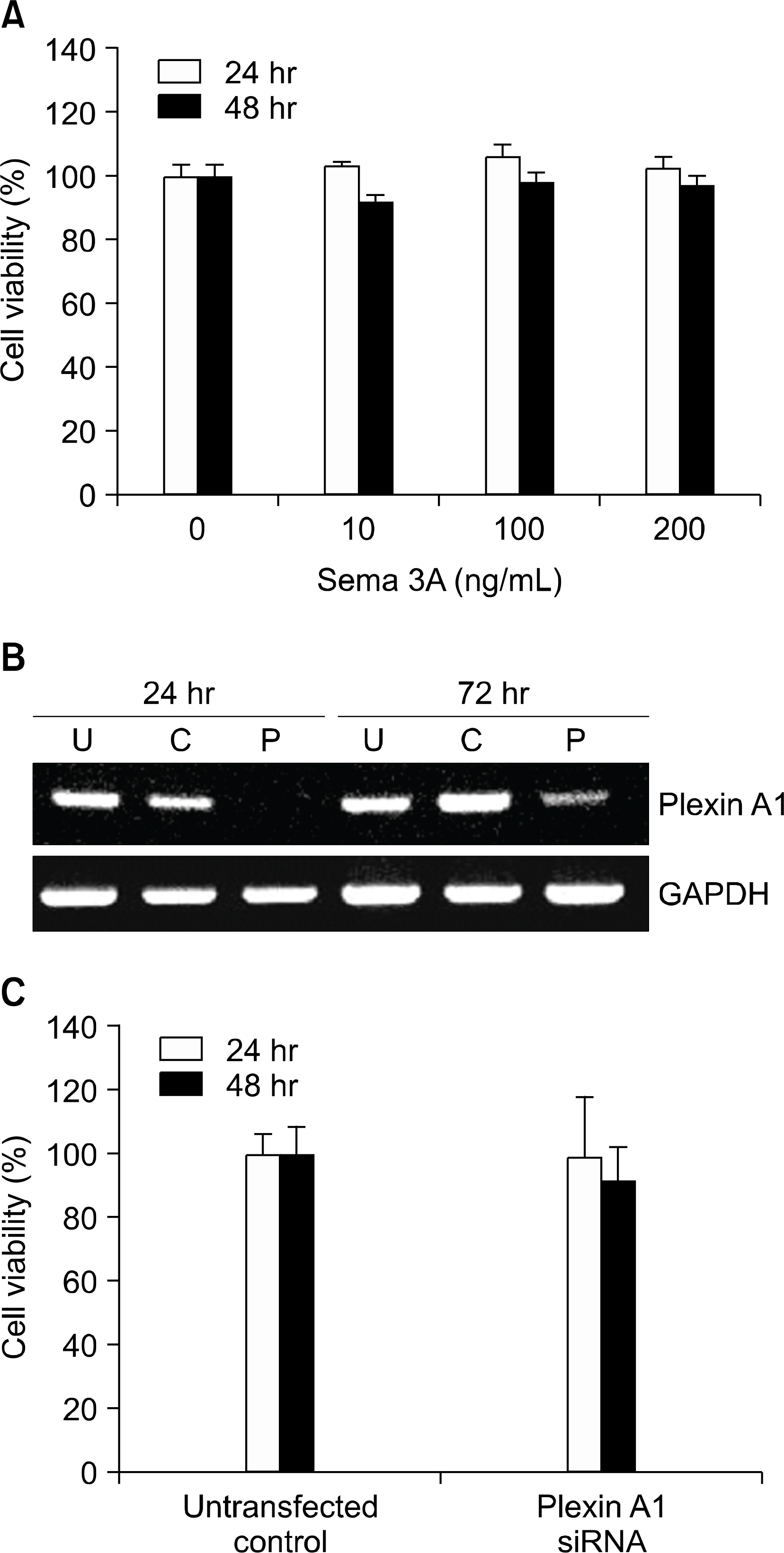
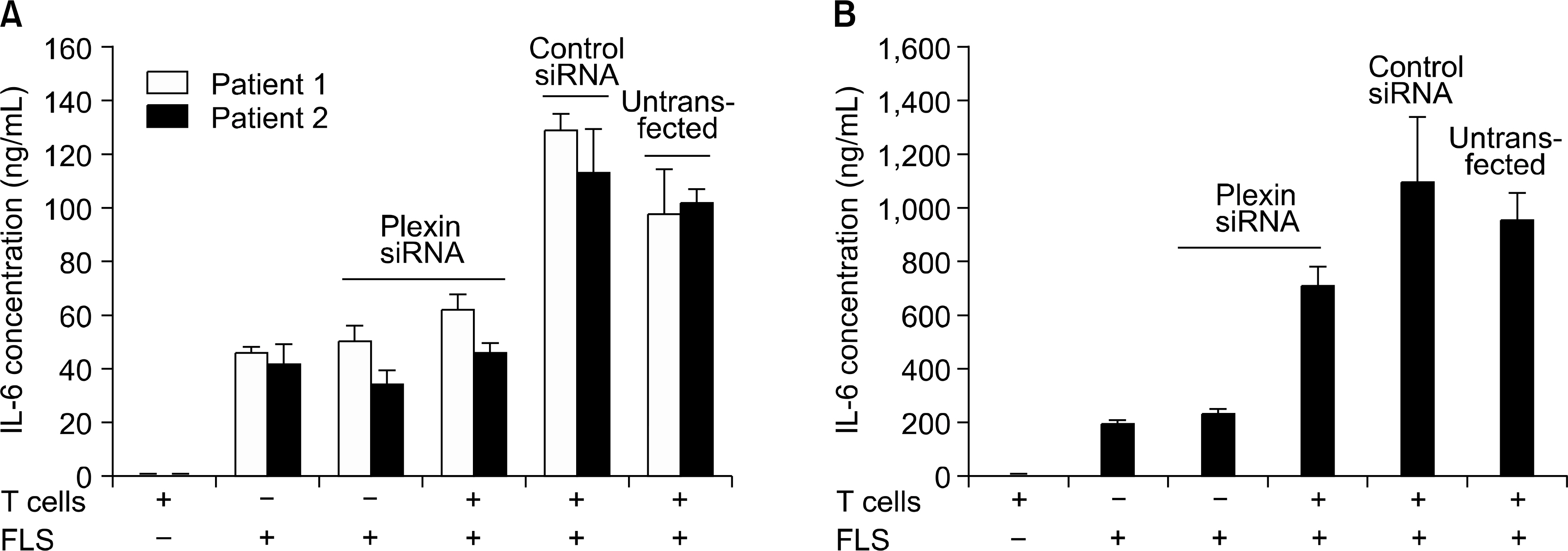
Plexin A1 was highly expressed in the lining layer of synovium and cultured FLS of RA patients. In RA FLS, basal expression of plexin A1 was higher than osteoarthritis FLS. On immunocytochemical staining, plexin A1 was co-expressed with neuropilin-1 in RA FLS. Semaphorin 3A (10 to 200 ng/mL), a specific ligand for neuropilin-1/plexin A1 complex, did not affect viability of RA FLS. The down regulation of plexin A1 mRNA by siRNA did not cause cell death, either. Co-culture of FLS with RA T cells, isolated from peripheral blood or synovial fluid, caused an increase in the productions of IL-6 and MCP-1 from FLS, but which were blocked by down-regulating plexin A1 transcripts using siRNA method.
Conclusion
These data suggest that enhanced expression of plexin A1 in RA FLS may elicit over-production of IL-6 and MCP-1, and thereby contribute to perpetuation of chronic inflammation in RA.
Keyword
MeSH Terms
-
Arthritis, Rheumatoid
Blotting, Western
Cell Communication
Cell Death
Cell Survival
Coculture Techniques
Down-Regulation
Enzyme-Linked Immunosorbent Assay
Humans
Immunohistochemistry
Inflammation
Interleukin-6
Monocytes
Neuropilin-1
Osteoarthritis
RNA, Messenger
RNA, Small Interfering
Semaphorin-3A
Sincalide
Synovial Fluid
Synovial Membrane
T-Lymphocytes
Interleukin-6
Neuropilin-1
RNA, Messenger
RNA, Small Interfering
Semaphorin-3A
Sincalide
Figure
Reference
-
1). Feldmann M., Brennan FM., Maini RN. Rheumatoid arthritis. Cell. 1996. 85:307–10.
Article2). Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996. 39:1781–90.3). Koch A. Angiogenesis: implications for rheumatoid arthritis. Arthritis Rheum. 1998. 41:951–62.
Article4). Dvorak HF., Brown LF., Detmar M., Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hypermeability and angiogenesis. Am J Pathol. 1995. 146:1029–39.5). Firestein GS. Starving the synovium: angiogenesis and inflammation in rheumatoid arthritis. J Clin Invest. 1999. 103:3–4.
Article6). Lee SS., Joo YS., Kim WU., Min DJ., Min JK., Park SH, et al. Vascular endothelial growth factor levels in the serum and synovial fluid of patients with rheumatoid arthritis. Clin Exp Rheumatol. 2001. 19:321–4.7). Kim WU., Kang SS., Yoo SA., Hong KH., Bae DG., Lee MS, et al. Interaction of vascular endothelial growth factor165 with neuropilin-1 protects rheumatoid synoviocytes from apoptotic death by regulating Bcl-2 expression and Bax translocation. J Immunol. 2006. 177:5727–35.8). Kikutani H., Kumanogoh A. Semaphorins in interactions between T cells and antigen-presenting cells. Nat Rev Immunol. 2003. 3:159–67.
Article9). Narazaki M., Tosato G. Ligand-induced internalization selects use of common receptor neuropilin-1 by VEGF165 and semaphorin3A. Blood. 2006. 107:3892–901.
Article10). Takahashi T., Fournier A., Nakamura F., Wang LH., Murakami Y., Kalb RG, et al. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999. 99:59–69.
Article11). Tordjman R., Lepelletier Y., Lemarchandel V., Cambot M., Gaulard P., Hermine ᄋ, et al. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat Immunol. 2002. 3:477–82.
Article12). Wong AW., Brickey WJ., Taxman DJ., van Deventer HW., Reed W., Gao JX, et al. CIITA-regulated plexin-A1 affects T-cell-dendritic cell interactions. Nat Immunol. 2003. 4:891–8.
Article13). Takegahara N., Takamatsu H., Toyofuku T., Tsujimura T., ᄋkuno T., Yukawa K, et al. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nature Cell Biology. 2006. 8:615–22.
Article14). Yoo SA., Bae DG., Ryoo JW., Kim HR., Park GS., Cho CS, et al. Arginine-rich anti-vascular endothelial growth factor (anti-VEGF) hexapeptide inhibits collagen-induced arthritis and VEGF-stimulated productions of TNF-alpha and IL-6 by human monocytes. J Immunol. 2005. 174:5846–55.15). Guan F., Villegas G., Teichman J., Mundel P., Tufro A. Autocrine class 3 semaphorin system regulates slit diaphragm proteins and podocyte survival. Kidney Int. 2006. 69:1564–9.
Article16). Yamamura Y., Gupta R., Morita Y., He X., Pai R., Endres J, et al. Effector function of resting T cells: activation of synovial fibroblasts. J Immunol. 2001. 166:2270–5.
Article17). Min DJ., Cho ML., Lee SH., Min SY., Kim WU., Min JK, et al. Augmented production of chemokines by the interaction of type II collagen-reactive T cells with rheumatoid synovial fibroblasts. Arthritis Rheum. 2004. 50:1146–55.
Article18). Eun SY., ᄋ,Connor BP., Wong AW., van Deventer HW., Taxman DJ., Reed W, et al. Cutting edge: rho activation and actin polarization are dependent on plexin-A1 in dendritic cells. J Immunol. 2006. 177:4271–5.
Article19). Negishi M., ᄋinuma I., Katoh H. Plexins: axon guidance and signal transduction. Cell Mol Life Sci. 2005. 62:1363–71.
Article20). Kruger RP., Aurandt J., Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005. 6:789–800.
Article21). Miller LE., Weidler C., Falk W., Angele P., Schaum-burger J., Scholmerich J, et al. Increased prevalence of semaphorin 3C, a repellent of sympathetic nerve fibers, in the synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 2004. 50:1156–63.
Article22). Mangasser-Stephan K., Dooley S., Welter C., Mutschler W., Hanselmann RG. Identification of human semaphorin E gene expression in rheumatoid synovial cells by mRNA differential display. Biochem Biophys Res Commun. 1997. 234:153–6.
Article23). Lundy SK., Sarkar S., Tesmer LA., Fox DA. Cells of the synovium in rheumatoid arthritis. Tlymphocytes. Arthritis Res Ther. 2007. 9:202.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression and Function of Calcineurin in Inflammatory Arthritis
- The Effects of Interleukin-17 on Production of Matrix Metalloproteinase-3 in Cultured Rheumatoid Arthritis Synoviocytes
- The Roles of Intercellular Adhesion Molecule I in T Cell Adhesion Tosynovial Cell in Patients with Rheumatoid Arthritis
- Caffeic Acid Phenethyl Ester Inhibits the PKC-Induced IL-6 Gene Expression in the Synoviocytes of Rheumatoid Arthritis Patients
- Regulation of Matrix Metalloproteinases and Tissue Inhibitor of Metalloproteinase-1 Expression by Hypoxia in Rheumatoid and Osteoarthritis Fibroblast-Like Synoviocytes