Anat Cell Biol.
2012 Mar;45(1):26-37. 10.5115/acb.2012.45.1.26.
Evidence of early involvement of apoptosis inducing factor-induced neuronal death in Alzheimer brain
- Affiliations
-
- 1Department of Brain Science, Graduate School, Daegu Gyeongbuk Institute of Science and Technology (DGIST), Daegu, Korea.
- 2Department of Anatomy and Neuroscience, Eulji University School of Medicine, Daejeon, Korea. tkbaik@eulji.ac.kr
- 3Department of Pediatrics, Yeungnam University College of Medicine, Daegu, Korea.
- KMID: 1447450
- DOI: http://doi.org/10.5115/acb.2012.45.1.26
Abstract
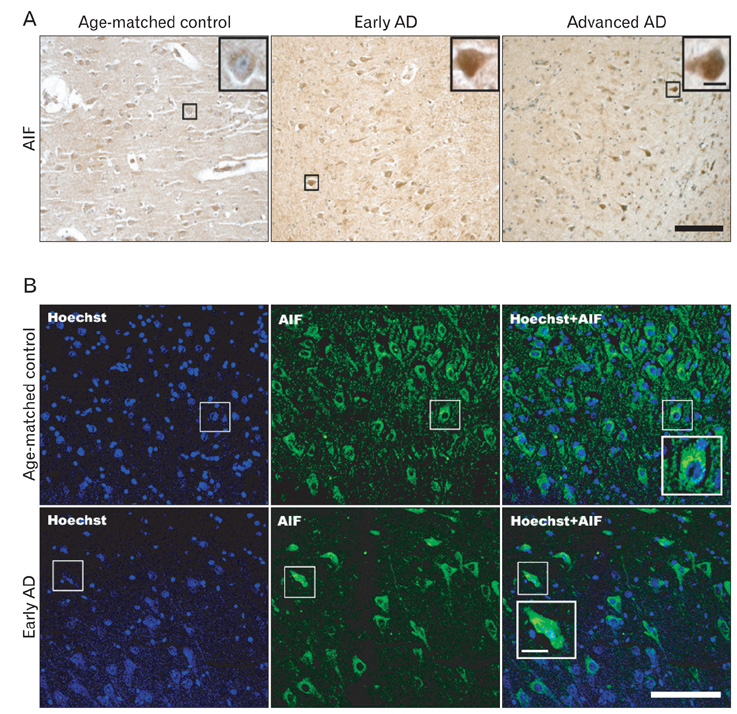
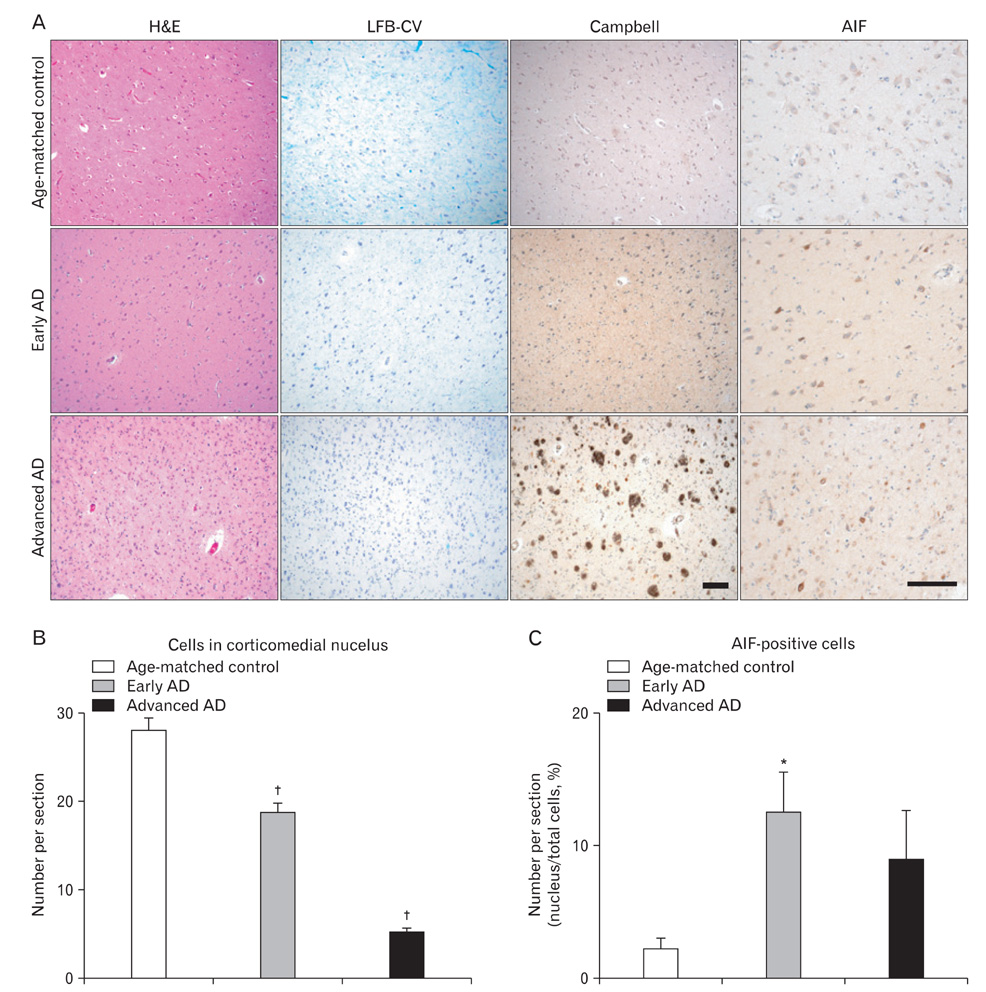
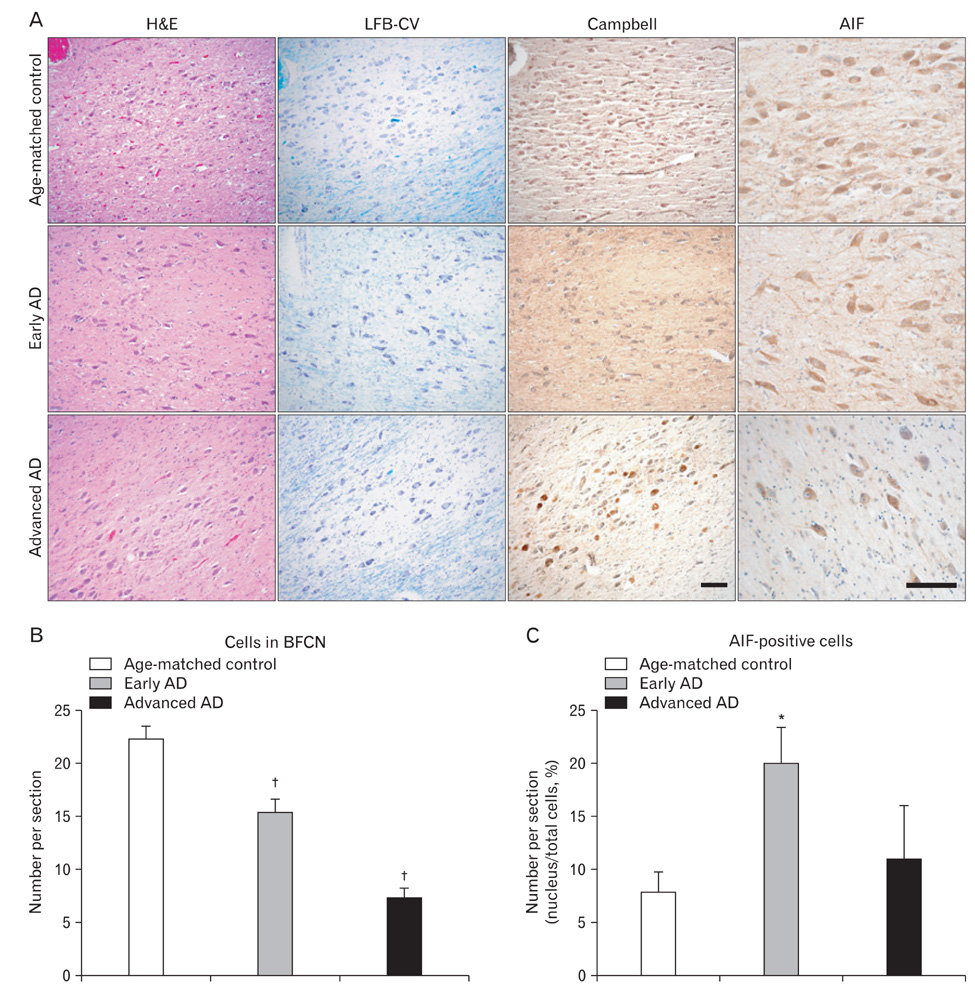
- Apoptosis inducing factor (AIF) has been proposed to act as a putative reactive oxygen species scavenger in mitochondria. When apoptotic cell death is triggered, AIF translocates to the nucleus, where it leads to nuclear chromatin condensation and large-scale DNA fragmentation which result in caspase-independent neuronal death. We performed this study to investigate the possibility that, in addition to caspase-dependent neuronal death, AIF induced neuronal death could be a cause of neuronal death in Alzheimer's disease (AD). We have found that AIF immunoreactivity was increased in the hippocampal pyramidal neurons in the Alzheimer brains compared to those of healthy, age-matched control brains. Nuclear AIF immunoreactivity was detected in the apoptotic pyramidal CA1 neurons at the early stage of AD and CA2 at the advanced stage. Nuclear AIF positive neurons were also observed in the amygdala and cholinergic neurons of the basal forebrain (BFCN) from the early stages of AD. The results of this study imply that AIF-induced apoptosis may contribute to neuronal death within the hippocampus, amygdala, and BFCN in early of AD.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Large-scale functional brain networks for consciousness
Myoung-Eun Han, Si-Young Park, Sae-Ock Oh
Anat Cell Biol. 2021;54(2):152-164. doi: 10.5115/acb.20.305.
Reference
-
1. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002. 297:353–356.2. Mudher A, Lovestone S. Alzheimer's disease: do tauists and baptists finally shake hands? Trends Neurosci. 2002. 25:22–26.3. Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004. 430:631–639.4. Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001. 58:1395–1402.5. Nunomura A, Chiba S. Avoidance of apoptosis in Alzheimer's Disease. J Alzheimers Dis. 2000. 2:59–60.6. Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001. 60:759–767.7. Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer's disease. J Neurosci. 1999. 19:1959–1964.8. Praticò D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001. 21:4183–4187.9. Lee HG, Castellani RJ, Zhu X, Perry G, Smith MA. Amyloid-beta in Alzheimer's disease: the horse or the cart? Pathogenic or protective? Int J Exp Pathol. 2005. 86:133–138.10. Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001. 411:342–348.11. Klein JA, Longo-Guess CM, Rossmann MP, Seburn KL, Hurd RE, Frankel WN, Bronson RT, Ackerman SL. The harlequin mouse mutation downregulates apoptosis-inducing factor. Nature. 2002. 419:367–374.12. Otera H, Ohsakaya S, Nagaura Z, Ishihara N, Mihara K. Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J. 2005. 24:1375–1386.13. Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999. 397:441–446.14. Reix S, Mechawar N, Susin SA, Quirion R, Krantic S. Expression of cortical and hippocampal apoptosis-inducing factor (AIF) in aging and Alzheimer's disease. Neurobiol Aging. 2007. 28:351–356.15. Yu W, Mechawar N, Krantic S, Quirion R. Evidence for the involvement of apoptosis-inducing factor-mediated caspase-independent neuronal death in Alzheimer disease. Am J Pathol. 2010. 176:2209–2218.16. Campbell SK, Switzer RC, Martin TL. Alzheimer's plaques and tangles: a controlled and enhanced silver staining method. Soc Neurosci Abstr. 1987. 13:678.17. Gallyas F. Silver staining of Alzheimer's neurofibrillary changes by means of physical development. Acta Morphol Acad Sci Hung. 1971. 19:1–8.18. Woo RS, Lee JH, Yu HN, Song DY, Baik TK. Expression of ErbB4 in the neurons of Alzheimer's disease brain and APP/PS1 mice, a model of Alzheimer's disease. Anat Cell Biol. 2011. 44:116–127.19. Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991. 1:213–216.20. Geula C, Mesulam MM. Terry RD, Katzman R, Blick KL, Sisodia SS, editors. Cholinergic systems in Alzheimer's disease. Alzheimer Disease. 1999. 2nd ed. New York: Raven Press;269–292.21. Jellinger KA. Cell death mechanisms in neurodegeneration. J Cell Mol Med. 2001. 5:1–17.22. Cotman CW, Su JH. Mechanisms of neuronal death in Alzheimer's disease. Brain Pathol. 1996. 6:493–506.23. Beal MF. Mitochondrial dysfunction and oxidative damage in Alzheimer's and Parkinson's diseases and coenzyme Q10 as a potential treatment. J Bioenerg Biomembr. 2004. 36:381–386.24. Galvan V, Chen S, Lu D, Logvinova A, Goldsmith P, Koo EH, Bredesen DE. Caspase cleavage of members of the amyloid precursor family of proteins. J Neurochem. 2002. 82:283–294.25. Takuma H, Tomiyama T, Kuida K, Mori H. Amyloid beta peptide-induced cerebral neuronal loss is mediated by caspase-3 in vivo. J Neuropathol Exp Neurol. 2004. 63:255–261.26. Zhao M, Su J, Head E, Cotman CW. Accumulation of caspase cleaved amyloid precursor protein represents an early neurodegenerative event in aging and in Alzheimer's disease. Neurobiol Dis. 2003. 14:391–403.27. Bertrand E, Brouillet E, Caillé I, Bouillot C, Cole GM, Prochiantz A, Allinquant B. A short cytoplasmic domain of the amyloid precursor protein induces apoptosis in vitro and in vivo. Mol Cell Neurosci. 2001. 18:503–511.28. Stadelmann C, Deckwerth TL, Srinivasan A, Bancher C, Brück W, Jellinger K, Lassmann H. Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer's disease: evidence for apoptotic cell death. Am J Pathol. 1999. 155:1459–1466.29. Yang DS, Kumar A, Stavrides P, Peterson J, Peterhoff CM, Pawlik M, Levy E, Cataldo AM, Nixon RA. Neuronal apoptosis and autophagy cross talk in aging PS/APP mice, a model of Alzheimer's disease. Am J Pathol. 2008. 173:665–681.30. Bahi N, Zhang J, Llovera M, Ballester M, Comella JX, Sanchis D. Switch from caspase-dependent to caspase-independent death during heart development: essential role of endonuclease G in ischemia-induced DNA processing of differentiated cardiomyocytes. J Biol Chem. 2006. 281:22943–22952.31. Wang H, Yu SW, Koh DW, Lew J, Coombs C, Bowers W, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Apoptosis-inducing factor substitutes for caspase executioners in NMDA-triggered excitotoxic neuronal death. J Neurosci. 2004. 24:10963–10973.32. Movsesyan VA, Stoica BA, Faden AI. MGLuR5 activation reduces beta-amyloid-induced cell death in primary neuronal cultures and attenuates translocation of cytochrome c and apoptosis-inducing factor. J Neurochem. 2004. 89:1528–1536.33. Zhang X, Chen J, Graham SH, Du L, Kochanek PM, Draviam R, Guo F, Nathaniel PD, Szabó C, Watkins SC, Clark RS. Intranuclear localization of apoptosis-inducing factor (AIF) and large scale DNA fragmentation after traumatic brain injury in rats and in neuronal cultures exposed to peroxynitrite. J Neurochem. 2002. 82:181–191.34. Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience. 1983. 10:1185–1201.35. Perry RH, Candy JM, Perry EK, Thompson J, Oakley AE. The substantia innominata and adjacent regions in the human brain: histochemical and biochemical observations. J Anat. 1984. 138(Pt 4):713–732.36. Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982. 215:1237–1239.37. Nagai T, McGeer PL, Peng JH, McGeer EG, Dolman CE. Choline acetyltransferase immunohistochemistry in brains of Alzheimer's disease patients and controls. Neurosci Lett. 1983. 36:195–199.38. Emre M, Heckers S, Mash DC, Geula C, Mesulam MM. Cholinergic innervation of the amygdaloid complex in the human brain and its alterations in old age and Alzheimer's disease. J Comp Neurol. 1993. 336:117–134.39. Grunthal E. Pathological analysis of the symptoms of Alzheimer's disease. Pscychiatr Neurol Wochenschr. 1928. 36:401–407.40. Hopper MW, Vogel FS. The limbic system in Alzheimer's disease: a neuropathologic investigation. Am J Pathol. 1976. 85:1–20.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of Apoptosis Inducing Factor in the Rat Brain with Ischemic Injury Induced by Middle Cerebral Artery Occlusion
- Alzheimer's Disease and Apoptosis
- Protective Effect of Fibroin BF-7 on Neuronal Cell Death in Alzheimer Model using Amyloid beta Peptide
- Involvement of Up-regulation of Death Receptors and Bim in Hispolon-mediated TNF-related Apoptosis-inducing Ligand Sensitization in Human Renal Carcinoma
- Caspase is Regulated by ROS in CT Induced Neuronal Cell Death