Lab Anim Res.
2013 Jun;29(2):113-126. 10.5625/lar.2013.29.2.113.
In vitro and in vivo study of effects of fermented soybean product (chungkookjang) on NGF secretion ability and NGF receptor signaling pathway
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources & Life Science, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- 2Department of Life Science and Environmental Biochemistry, College of Natural Resources & Life Science, Pusan National University, Miryang, Korea.
- 3Department of Food Science & Technology, College of Natural Resources & Life Science, Pusan National University, Miryang, Korea.
- KMID: 1431345
- DOI: http://doi.org/10.5625/lar.2013.29.2.113
Abstract
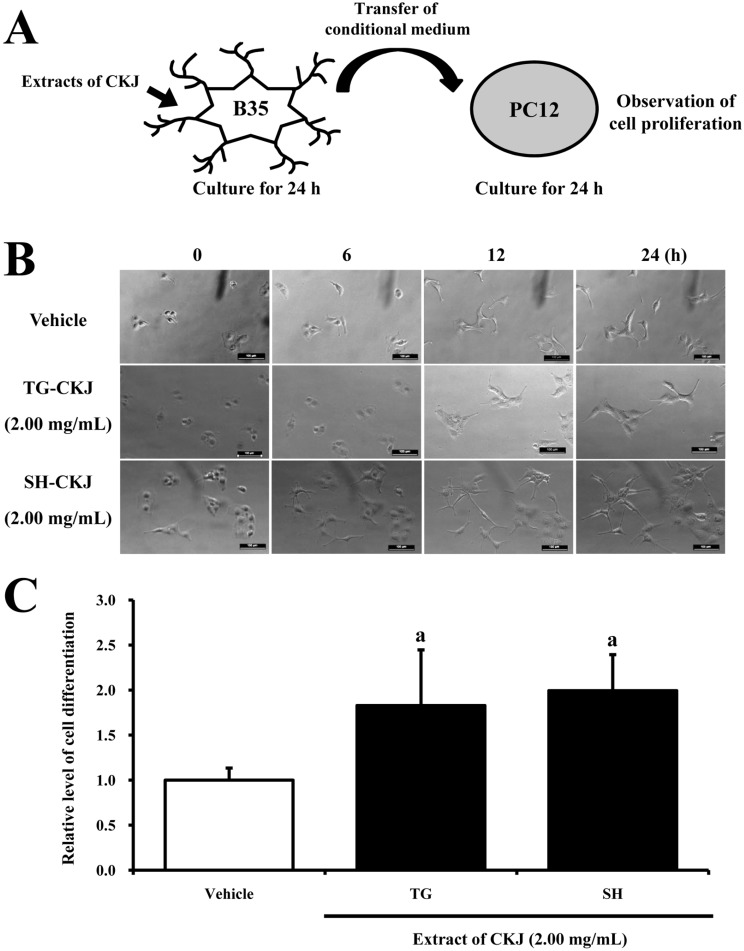
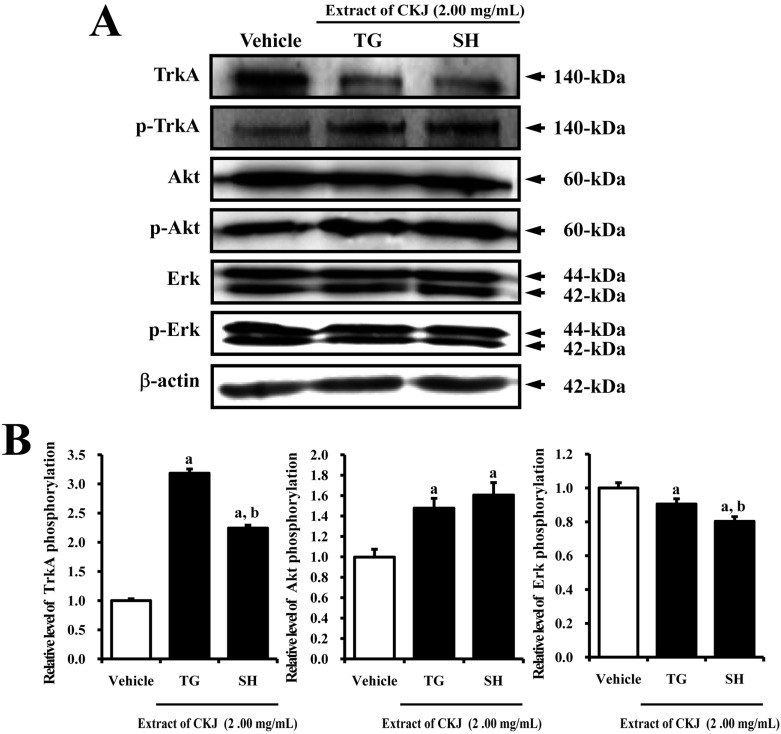
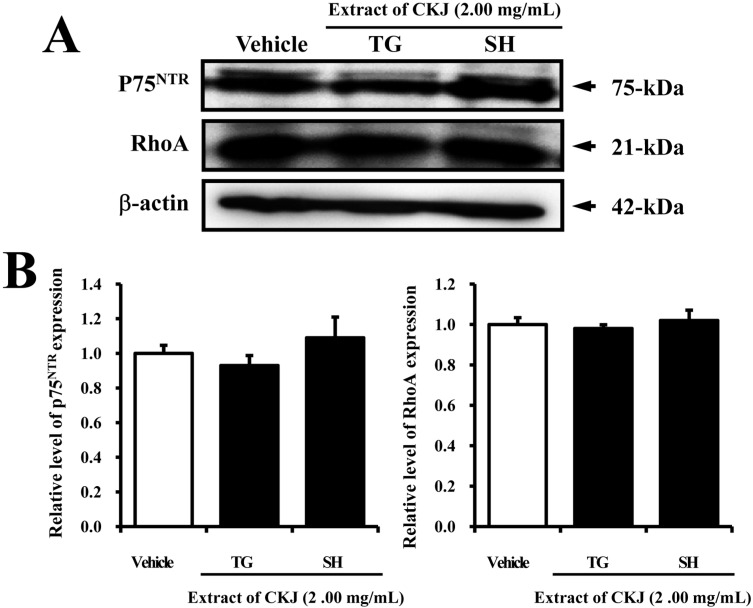
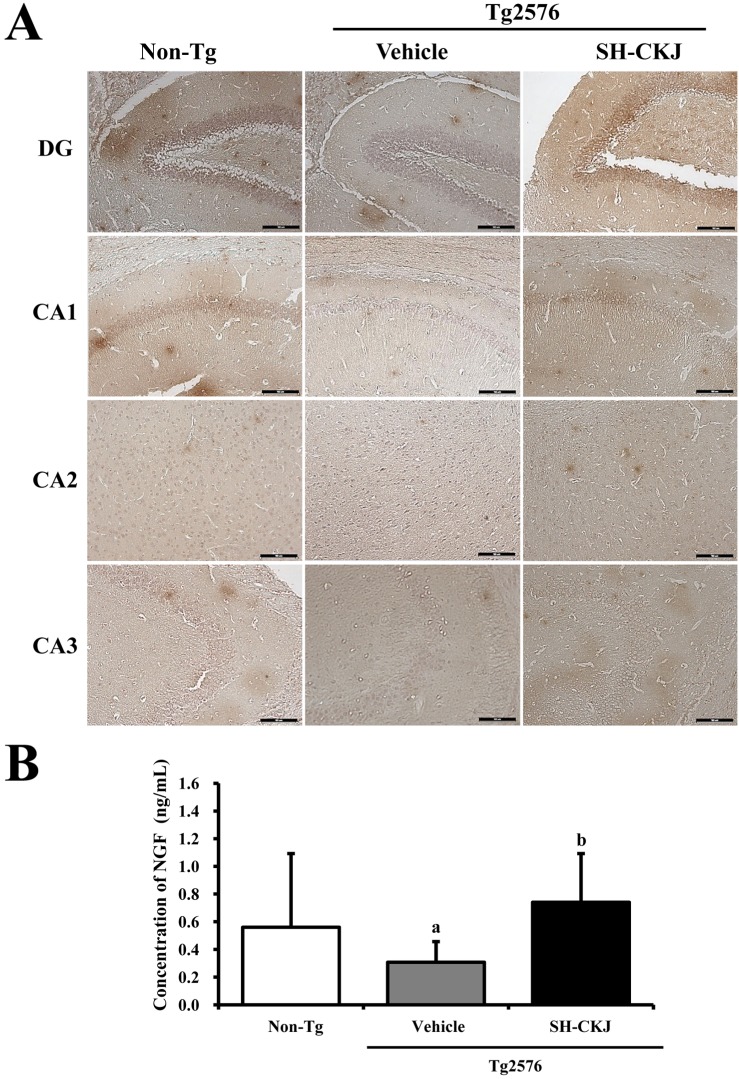
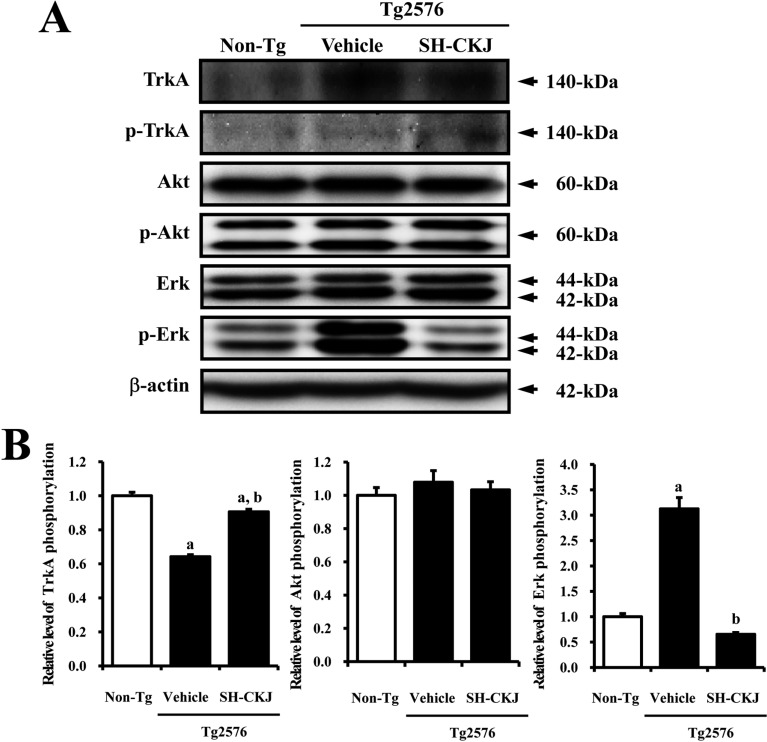
- In order to investigate the effects of a fermented soybean product (Chungkookjang, CKJ) on nerve growth factor (NGF) metabolism, NGF secretion ability and its related signaling pathway were analyzed in B35 neuronal cells and the Tg2576 mouse model of Alzheimer's disease (AD). In B35 cells, the concentration of NGF significantly increased upon treatment with Taegwang (TG)-CKJ and Shinhwa (SH)-CKJ extracts compared with vehicle. Further, a significant increase in PC12 cell length as well as the phsophorylation levels of TrkA and Akt, which are members of a high affinity NGF receptor signaling pathway, were observed after treatment with TG-CKJ and SH-CKJ conditional medium (CM). On the other hand, there was no difference in activation of the NGF receptor p75NTR signaling pathway between vehicle and all CKJ treated groups. In Tg2576 mice showing early stage of AD, the concentrations of NGF in the serum and brain were reduced compared with those in Non-Tg mice. Treatment of Tg2576 mice with SH-CKJ, which contains high concentrations of total flavonoids and phenolic compounds, for 8 weeks dramatically recovered the NGF level to that of Non-Tg mice. Furthermore, the low phosphorylation levels of TrkA and Erk in the NGF receptor TrkA signaling pathway were rapidly recovered to those of Non-Tg mice after SH-CKJ treatment in vehicle treated Tg2576 mice, whereas the phosphorylation level of Akt was maintained at a constant level. These results suggest that CKJ may stimulate NGF secretion ability as well as the NGF receptor TrkA signaling pathway in PC12 cells and Tg2576 mice.
Keyword
MeSH Terms
Figure
Reference
-
1. Mölsä PK, Marttila RJ, Rinne UK. Long-term survival and predictors of mortality in Alzheimer's disease and multi-infarct dementia. Acta Neurol Scand. 1995; 91(3):159–164. PMID: 7793228.
Article2. Tabert MH, Liu X, Doty RL, Serby M, Zamora D, Pelton GH, Marder K, Albers MW, Stern Y, Devanand DP. A 10-item smell identification scale related to risk for Alzheimer's disease. Ann Neurol. 2005; 58(1):155–160. PMID: 15984022.
Article3. Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology. 2004; 62(11):1984–1989. PMID: 15184601.
Article4. Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH. Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cereb Cortex. 1994; 4(2):138–150. PMID: 8038565.
Article5. Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci. 2006; 26(27):7212–7221. PMID: 16822978.
Article6. Turner PR, O'Connor K, Tate WP, Abraham WC. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol. 2003; 70(1):1–32. PMID: 12927332.
Article7. Pohanka M. Cholinesterases, a target of pharmacology and toxicology. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011; 155(3):219–229. PMID: 22286807.
Article8. Stahl SM. The new cholinesterase inhibitors for Alzheimer's disease, Part 2: illustrating their mechanisms of action. J Clin Psychiatry. 2000; 61(11):813–814. PMID: 11105732.
Article9. Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006; 5(2):160–170. PMID: 16424917.
Article10. Lee JJ, Lee DS, Kim HB. Fermentation patterns of Chungkookjang and Kanjang by Bacillus licheniformis B1. Korean J Microbiol. 1999; 35:296–301.11. Su CL, Wu CJ, Chen FN, Wang BJ, Sheu SR, Won SJ. Supernatant of bacterial fermented soybean induces apoptosis of human hepatocellular carcinoma Hep 3B cells via activation of caspase 8 and mitochondria. Food Chem Toxicol. 2007; 45(2):303–314. PMID: 17030378.
Article12. Nakajima N, Nozaki N, Ishihara K, Ishikawa A, Tsuji H. Analysis of isoflavone content in tempeh, a fermented soybean, and preparation of a new isoflavone-enriched tempeh. J Biosci Bioeng. 2005; 100(6):685–687. PMID: 16473782.
Article13. Kwon DY, Jang JS, Lee JE, Kim YS, Shin DH, Park S. The isoflavonoid aglycone-rich fractions of Chungkookjang, fermented unsalted soybeans, enhance insulin signaling and peroxisome proliferator-activated receptor-gamma activity in vitro. Biofactors. 2006; 26(4):245–258. PMID: 17119271.14. Kim DJ, Jeong YJ, Kwon JH, Moon KD, Kim HJ, Jeon SM, Lee MK, Park YB, Choi MS. Beneficial effect of chungkukjang on regulating blood glucose and pancreatic beta-cell functions in C75BL/KsJ-db/db mice. J Med Food. 2008; 11(2):215–223. PMID: 18598161.15. Kwon DY, Daily JW 3rd, Kim HJ, Park S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr Res. 2010; 30(1):1–13. PMID: 20116654.
Article16. Choi YH, Lim H, Heo MY, Kwon DY, Kim HP. Anti-inflammatory activity of the ethanol extract of Chungkukjang, Korean fermented bean: 5-lipoxygenase inhibition. J Med Food. 2008; 11(3):539–543. PMID: 18800904.
Article17. Kim HB, Lee HS, Kim SJ, Yoo HJ, Hwang JS, Chen G, Youn HJ. Ethanol extract of fermented soybean, Chungkookjang, inhibits the apoptosis of mouse spleen, and thymus cells. J Microbiol. 2007; 45(3):256–261. PMID: 17618232.18. Hwang DY, Chae KR, Kang TS, Hwang JH, Lim CH, Kang HK, Goo JS, Lee MR, Lim HJ, Min SH, Cho JY, Hong JT, Song CW, Paik SG, Cho JS, Kim YK. Alterations in behavior, amyloid beta-42, caspase-3, and Cox-2 in mutant PS2 transgenic mouse model of Alzheimer's disease. FASEB J. 2002; 16(8):805–813. PMID: 12039862.19. Prajapati KD, Sharma SS, Roy N. Upregulation of albumin expression in focal ischemic rat brain. Brain Res. 2010; 1327:118–124. PMID: 20193666.
Article20. Tsui-Pierchala BA, Ginty DD. Characterization of an NGF-P-TrkA retrograde-signaling complex and age-dependent regulation of TrkA phosphorylation in sympathetic neurons. J Neurosci. 1999; 19(19):8207–8218. PMID: 10493722.
Article21. Barres BA, Barde Y. Neuronal and glial cell biology. Curr Opin Neurobiol. 2000; 10(5):642–648. PMID: 11084327.
Article22. Levi-Montalcini R. The nerve growth factor: thirty-five years later. EMBO J. 1987; 6(5):1145–1154. PMID: 3301324.
Article23. Fiore M, Chaldakov GN, Aloe L. Nerve growth factor as a signaling molecule for nerve cells and also for the neuroendocrine-immune systems. Rev Neurosci. 2009; 20(2):133–145. PMID: 19774790.
Article24. Diaz Brinton R, Yamazaki RS. Advances and challenges in the prevention and treatment of Alzheimer's disease. Pharm Res. 1998; 15(3):386–398. PMID: 9563067.25. Hur J, Lee P, Kim J, Kim AJ, Kim H, Kim SY. Induction of nerve growth factor by butanol fraction of Liriope platyphylla in C6 and primary astrocyte cells. Biol Pharm Bull. 2004; 27(8):1257–1260. PMID: 15305032.26. Choi JG, Moon M, Jeong HU, Kim MC, Kim SY, Oh MS. Cistanches Herba enhances learning and memory by inducing nerve growth factor. Behav Brain Res. 2011; 216(2):652–658. PMID: 20849880.
Article27. Uezato T, Sato E, Miura N. Screening of natural medicines that efficiently activate neurite outgrowth in PC12 cells in C2C12-cultured medium. Biomed Res. 2012; 33(1):25–33. PMID: 22361883.
Article28. Pan Y, Anthony M, Clarkson TB. Evidence for up-regulation of brain-derived neurotrophic factor mRNA by soy phytoestrogens in the frontal cortex of retired breeder female rats. Neurosci Lett. 1999; 261(1-2):17–20. PMID: 10081916.
Article29. Nakajima K, Niisato N, Marunaka Y. Genistein enhances the NGF-induced neurite outgrowth. Biomed Res. 2011; 32(5):351–356. PMID: 22033305.
Article30. Nakajima K, Niisato N, Marunaka Y. Quercetin stimulates NGF-induced neurite outgrowth in PC12 cells via activation of Na(+)/K(+)/2Cl(-) cotransporter. Cell Physiol Biochem. 2011; 28(1):147–156. PMID: 21865857.31. Perini G, Della-Bianca V, Politi V, Della Valle G, Dal-Pra I, Rossi F, Armato U. Role of p75 neurotrophin receptor in the neurotoxicity by beta-amyloid peptides and synergistic effect of inflammatory cytokines. J Exp Med. 2002; 195(7):907–918. PMID: 11927634.32. Russo C, Dolcini V, Salis S, Venezia V, Zambrano N, Russo T, Schettini G. Signal transduction through tyrosine-phosphorylated C-terminal fragments of amyloid precursor protein via an enhanced interaction with Shc/Grb2 adaptor proteins in reactive astrocytes of Alzheimer's disease brain. J Biol Chem. 2002; 277(38):35282–35288. PMID: 12084708.
Article33. Kang TH, Kim SY. Comparison of Nerve Growth Factor Induction by Butanol Fraction of Liriope platyphylla and Ophiopogon japonicas. Korean J Pharmacogn. 2008; 39(2):75–79.34. Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996; 274(5284):99–102. PMID: 8810256.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytotoxicity on Human Cancer Cells and Antitumorigenesis of Chungkookjang, a Fermented Soybean Product, in DMBA-Treated Rats
- Effects of Red Liriope platyphylla on NGF secretion ability, NGF receptor signaling pathway and gamma-secretase components in NSE/hAPPsw transgenic mice expressing Alzheimer's Disease
- Norepinephrine/β2 -Adrenergic Receptor Pathway Promotes the Cell Proliferation and Nerve Growth Factor Production in Triple-Negative Breast Cancer
- Precautionary effects of Red Liriope platyphylla on NGF secretion and Abeta42 deposition under the preclinical stage of Alzheimer's disease in Tg2576 mice
- Nerve Growth Factor Stimulates Glioblastoma Proliferation through Notch1 Receptor Signaling