J Korean Neurosurg Soc.
2018 Jul;61(4):441-449. 10.3340/jkns.2017.0219.
Nerve Growth Factor Stimulates Glioblastoma Proliferation through Notch1 Receptor Signaling
- Affiliations
-
- 1Department of Neurosurgery, Hallym University Sacred Heart Hospital, Anyang, Korea. nscib71@gmail.com
- 2Department of Neurosurgery, Dongtan Sacred Heart Hospital, Hwaseong, Korea.
- 3Department of Obstetrics and Gynecology, Hallym University Sacred Heart Hospital, Anyang, Korea.
- KMID: 2417319
- DOI: http://doi.org/10.3340/jkns.2017.0219
Abstract
OBJECTIVE
Notch receptors are heterodimeric transmembrane proteins that regulate cell fate, such as differentiation, proliferation, and apoptosis. Dysregulated Notch pathway signaling has been observed in glioblastomas, as well as in other human malignancies. Nerve growth factor (NGF) is essential for cell growth and differentiation in the nervous system. Recent reports suggest that NGF stimulates glioblastoma proliferation. However, the relationship between NGF and Notch1 in glioblastomas remains unknown. Therefore, we investigated expression of Notch1 in a glioblastoma cell line (U87-MG), and examined the relationship between NGF and Notch1 signaling.
METHODS
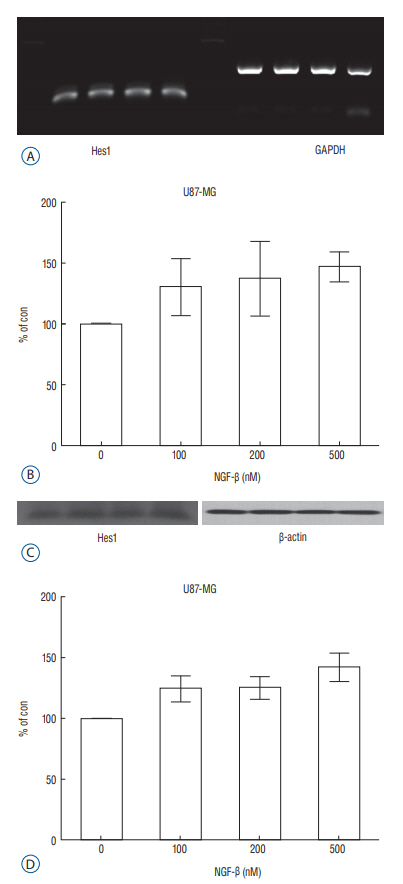
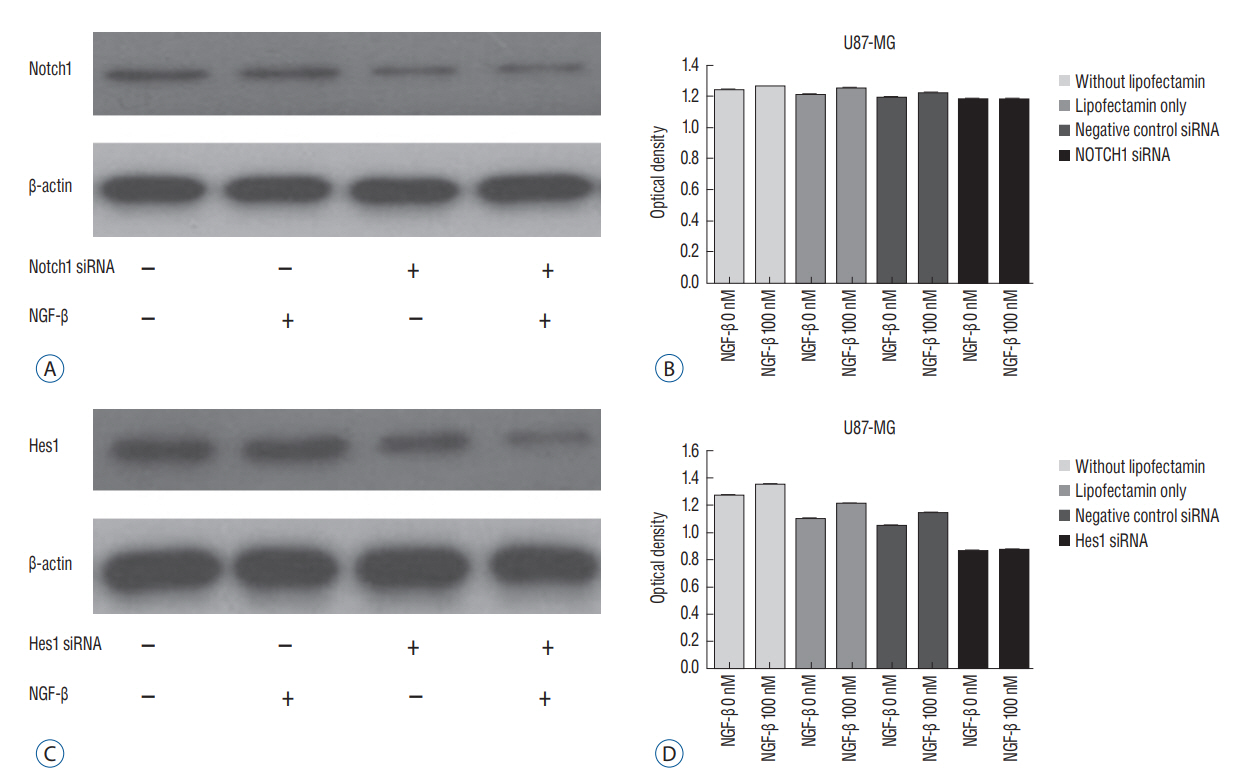
We evaluated expression of Notch1 in human glioblastomas and normal brain tissues by immunohistochemical staining. The effect of NGF on glioblastoma cell line (U87-MG) was evaluated by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. To evaluate the relationship between NGF and Notch1 signaling, Notch1 and Hes1 expression were evaluated by reverse transcription polymerase chain reaction (RT-PCR) and Western blot analysis, respectively. To confirm the effects of NGF on Notch1 signaling, Notch1 and Hes1 small interfering RNAs (siRNAs) were used.
RESULTS
In immunohistochemistry, Notch1 expression was higher in glioblastoma than in normal brain tissue. MTT assay showed that NGF stimulates U87-MG cells in a dose-dependent manner. RT-PCR and Western blot analysis demonstrated that Notch1 and Hes1 expression were increased by NGF in a dose-dependent manner. After transfection with Notch1 and Hes1 siRNAs, there was no significant difference between controls and 100 nM NGF-β, which means that U87-MG cell proliferation was suppressed by Notch1 and Hes1 siRNAs.
CONCLUSION
These results indicate that NGF stimulates glioblastoma cell proliferation via Notch1 signaling through Hes 1.
Keyword
MeSH Terms
-
Apoptosis
Blotting, Western
Brain
Cell Line
Cell Proliferation
Glioblastoma*
Humans
Immunohistochemistry
Nerve Growth Factor*
Nervous System
Polymerase Chain Reaction
Receptor, Notch1*
Receptors, Notch
Reverse Transcription
RNA, Small Interfering
Transfection
Nerve Growth Factor
RNA, Small Interfering
Receptor, Notch1
Receptors, Notch
Figure
Cited by 1 articles
-
Propranolol Inhibits the Proliferation of Human Glioblastoma Cell Lines through Notch1 and Hes1 Signaling System
Hyun Sik Kim, Young Han Park, Heui Seung Lee, Mi Jung Kwon, Joon Ho Song, In Bok Chang
J Korean Neurosurg Soc. 2021;64(5):716-725. doi: 10.3340/jkns.2021.0068.
Reference
-
References
1. Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 284:770–776. 1999.
Article2. Aster JC, Pear WS. Notch signaling in leukemia. Curr Opin Hematol. 8:237–244. 2001.
Article3. Bigas A, Martin DI, Milner LA. Notch1 and Notch2 inhibit myeloid differentiation in response to different cytokines. Mol Cell Biol. 18:2324–2333. 1998.
Article4. Bolós V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 28:339–363. 2007.
Article5. Brown MC, Staniszewska I, Lazarovici P, Tuszynski GP, Del Valle L, Marcinkiewicz C. Regulatory effect of nerve growth factor in alpha9beta1 integrin-dependent progression of glioblastoma. Neuro Oncol. 10:968–980. 2008.
Article6. Chao MV, Hempstead BL. p75 and Trk: a two-receptor system. Trends Neurosci. 18:321–326. 1995.
Article7. Chen J, Kesari S, Rooney C, Strack PR, Chen J, Shen H, et al. Inhibition of Notch signaling blocks growth of glioblastoma cell lines and tumor neurospheres. Genes Cancer. 1:822–835. 2010.
Article8. Cheung HC, Corley LJ, Fuller GN, McCutcheon IE, Cote GJ. Polypyrimidine tract binding protein and notch1 are independently re-expressed in glioma. Mod Pathol. 19:1034–1041. 2006.
Article9. Faux CH, Turnley AM, Epa R, Cappai R, Bartlett PF. Interactions between fibroblast growth factors and notch regulate neuronal differentiation. J Neurosci. 21:5587–5596. 2001.
Article10. Giraud S, Loum E, Bessette B, Mathonnet M, Lalloué F. P75 neurotrophin receptor is sequestered in the Golgi apparatus of the U-87 MG human glioblastoma cell line. Int J Oncol. 38:391–399. 2011.
Article11. Goretzki PE, Wahl RA, Becker R, Koller C, Branscheid D, Grussendorf M, et al. Nerve growth factor (NGF) sensitizes human medullary thyroid carcinoma (hMTC) cells for cytostatic therapy in vitro. Surgery. 102:1035–1042. 1987.12. Greenwald I. LIN-12/Notch signaling: lessons from worms and flies. Genes Dev. 12:1751–1762. 1998.13. Jang MS, Zlobin A, Kast WM, Miele L. Notch signaling as a target in multimodality cancer therapy. Curr Opin Mol Ther. 2:55–65. 2000.14. Jian Q, Tao Z, Li Y, Yin ZQ. Acute retinal injury and the relationship between nerve growth factor, notch1 transcription and short-lived dedifferentiation transient changes of mammalian Müller cells. Vision Res. 110(Pt A):107–117. 2015.
Article15. Jundt F, Anagnostopoulos I, Förster R, Mathas S, Stein H, Dörken B. Activated Notch1 signaling promotes tumor cell proliferation and survival in Hodgkin and anaplastic large cell lymphoma. Blood. 99:3398–3403. 2002.
Article16. Kanu OO, Mehta A, Di C, Lin N, Bortoff K, Bigner DD, et al. Glioblastoma multiforme: a review of therapeutic targets. Expert Opin Ther Targets. 13:701–718. 2009.
Article17. Kopan R, Ilagan MX. The canonical notch signaling pathway: unfolding the activation mechanism. Cell. 137:216–233. 2009.
Article18. Lawn S, Krishna N, Pisklakova A, Qu X, Fenstermacher DA, Fournier M, et al. Neurotrophin signaling via TrkB and TrkC receptors promotes the growth of brain tumor-initiating cells. J Biol Chem. 290:3814–3824. 2015.
Article19. Leong KG, Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood. 107:2223–2233. 2006.
Article20. Li J, Cui Y, Gao G, Zhao Z, Zhang H, Wang X. Notch1 is an independent prognostic factor for patients with glioma. J Surg Oncol. 103:813–817. 2011.
Article21. Liu Y, Su C, Shan Y, Yang S, Ma G. Targeting notch1 inhibits invasion and angiogenesis of human breast cancer cells via inhibition nuclear factorkappaB signaling. Am J Transl Res. 8:2681–2692. eCollection 2016.22. Miele L, Golde T, Osborne B. Notch signaling in cancer. Curr Mol Med. 6:905–918. 2006.
Article23. Miele L, Miao H, Nickoloff BJ. Notch signaling as a novel cancer therapeutic target. Curr Cancer Drug Targets. 6:313–323. 2006.
Article24. Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 33:416–421. 2003.
Article25. Oelmann E, Sreter L, Schuller I, Serve H, Koenigsmann M, Wiedenmann B, et al. Nerve growth factor stimulates clonal growth of human lung cancer cell lines and a human glioblastoma cell line expressing high-affinity nerve growth factor binding sites involving tyrosine kinase signaling. Cancer Res. 55:2212–2219. 1995.26. Park YH, Kim SJ, Jeong BH, Herzog TJ, Wright J, Kitajewski J, et al. Follicular stimulating hormone enhances Notch 1 expression in SK-OV-3 ovarian cancer cells. J Gynecol Oncol. 21:119–124. 2010.
Article27. Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 9:157–173. 2006.
Article28. Purow BW, Haque RM, Noel MW, Su Q, Burdick MJ, Lee J, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 65:2353–2363. 2005.
Article29. Qian XQ, Chen LL, Cheng Q, Tian Y, Luo XF, Wan XY. Inhibition of Notch 1 receptor influenced the differentiation of Lin-CD45RA-dendritic cell precursors within ovarian carcinoma microenvironment. BMC Immunol. 17:14. 2016.
Article30. Rakowicz-Szulczyńska EM, Herlyn M, Koprowski H. Nerve growth factor receptors in chromatin of melanoma cells, proliferating melanocytes, and colorectal carcinoma cells in vitro. Cancer Res. 48(24 Pt 1):7200–7206. 1988.31. Rakowicz-Szulczynska EM, Reddy U, Vorbrodt A, Herlyn D, Koprowski H. Chromatin and cell surface receptors mediate melanoma cell growth response to nerve growth factor. Mol Carcinog. 4:388–396. 1991.
Article32. Revoltella RP, Butler RH. Nerve growth factor may stimulate either division or differentiation of cloned C1300 neuroblastoma cells in serumfree cultures. J Cell Physiol. 104:27–33. 1980.
Article33. Singer HS, Hansen B, Martinie D, Karp CL. Mitogenesis in glioblastoma multiforme cell lines: a role for NGF and its TrkA receptors. J Neurooncol. 45:1–8. 1999.34. Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 24:1217–1281. 2001.
Article35. Sriuranpong V, Borges MW, Ravi RK, Arnold DR, Nelkin BD, Baylin SB, et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res. 61:3200–3205. 2001.36. Ström A, Castella P, Rockwood J, Wagner J, Caudy M. Mediation of NGF signaling by post-translational inhibition of HES-1, a basic helix-loophelix repressor of neuronal differentiation. Genes Dev. 11:3168–3181. 1997.
Article37. Takei Y, Laskey R. Interpreting crosstalk between TNF-alpha and NGF: potential implications for disease. Trends Mol Med. 14:381–388. 2008.
Article38. Xu P, Yu S, Jiang R, Kang C, Wang G, Jiang H, et al. Differential expression of Notch family members in astrocytomas and medulloblastomas. Pathol Oncol Res. 15:703–710. 2009.
Article39. Yavropoulou MP, Maladaki A, Yovos JG. The role of Notch and Hedgehog signaling pathways in pituitary development and pathogenesis of pituitary adenomas. Hormones (Athens). 14:5–18. 2015.
Article40. Zlobin A, Jang M, Miele L. Toward the rational design of cell fate modifiers: Notch signaling as a target for novel biopharmaceuticals. Curr Pharm Biotechnol. 1:83–106. 2000.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Propranolol Inhibits the Proliferation of Human Glioblastoma Cell Lines through Notch1 and Hes1 Signaling System
- Differential Effect of Genistein and Calphostin C on Phospholipase C Activation and Cell Proliferation in T98G Human Glioblastoma and Hs 683 Human Glioma Cells
- Interaction of Wnt5a with Notch1 is Critical for the Pathogenesis of Psoriasis
- Insulin-Like Growth Factor 1 Actions in Developing Brain and the Interaction with Wnt Pathway
- The Role of Notch1 Signaling in Anaplastic Thyroid Carcinoma