Allergy Asthma Immunol Res.
2020 May;12(3):537-555. 10.4168/aair.2020.12.3.537.
An Alternative Dendritic Cell-Induced Murine Model of Asthma Exhibiting a Robust Th2/Th17-Skewed Response
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck surgery, Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- 2Department of Otorhinolaryngology, Yonsei University College of Medicine, Seoul, Korea. jhyoon@yuhs.ac
- 3Department of Microbiology, Yonsei University College of Medicine, Seoul, Korea.
- 4Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. sjshin@yuhs.ac
- 5Department of Life Science, Research Institute for Natural Sciences, Hanyang University College of Natural Sciences, Seoul, Korea.
- 6The Airway Mucus Institute, Yonsei University College of Medicine, Seoul, Korea.
- 7Global Research Laboratory for Allergic Airway Diseases, Seoul, Korea.
- 8Department of Microbiology, Institute for Immunology and Immunological Diseases, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2471235
- DOI: http://doi.org/10.4168/aair.2020.12.3.537
Abstract
- PURPOSE
Simple and reliable animal models of human diseases contribute to the understanding of disease pathogenesis as well as the development of therapeutic interventions. Although several murine models to mimic human asthma have been established, most of them require anesthesia, resulting in variability among test individuals, and do not mimic asthmatic responses accompanied by T-helper (Th) 17 and neutrophils. As dendritic cells (DCs) are known to play an important role in initiating and maintaining asthmatic inflammation, we developed an asthma model via adoptive transfer of allergen-loaded DCs.
METHODS
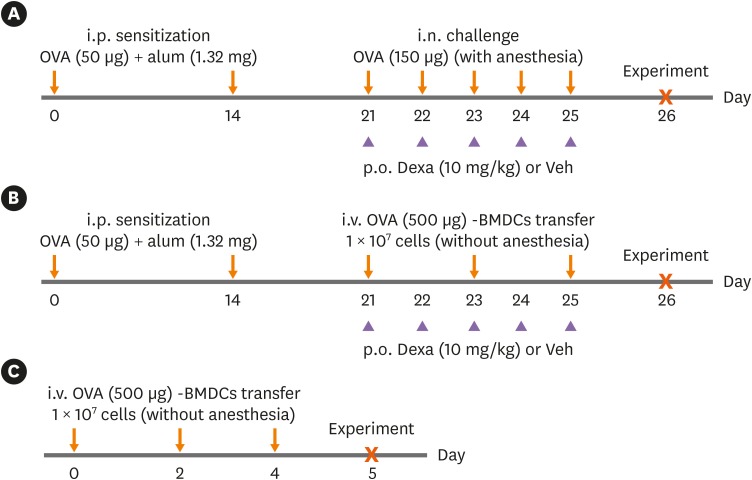
Ovalbumin (OVA)-loaded bone marrow-derived DCs (BMDCs) (OVA-BMDCs) were injected intravenously 3 times into non-anesthetized C57BL/6 mice after intraperitoneal OVA-sensitization.
RESULTS
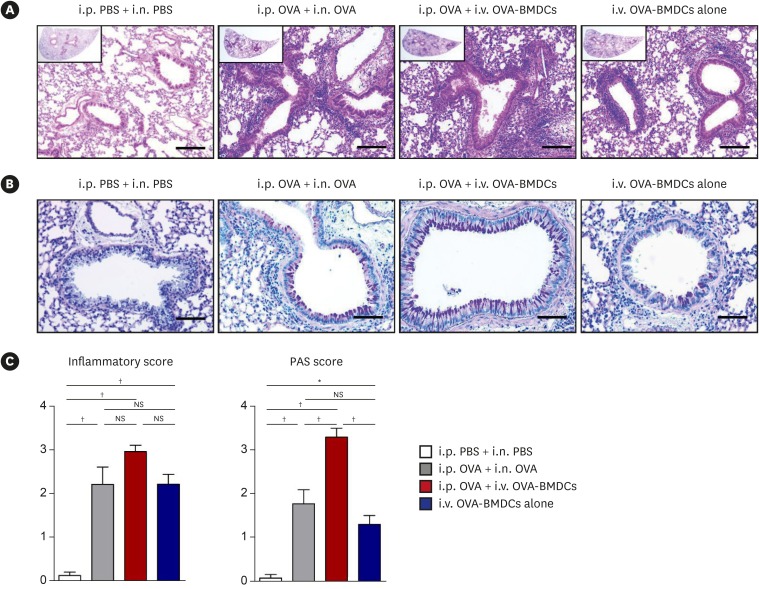
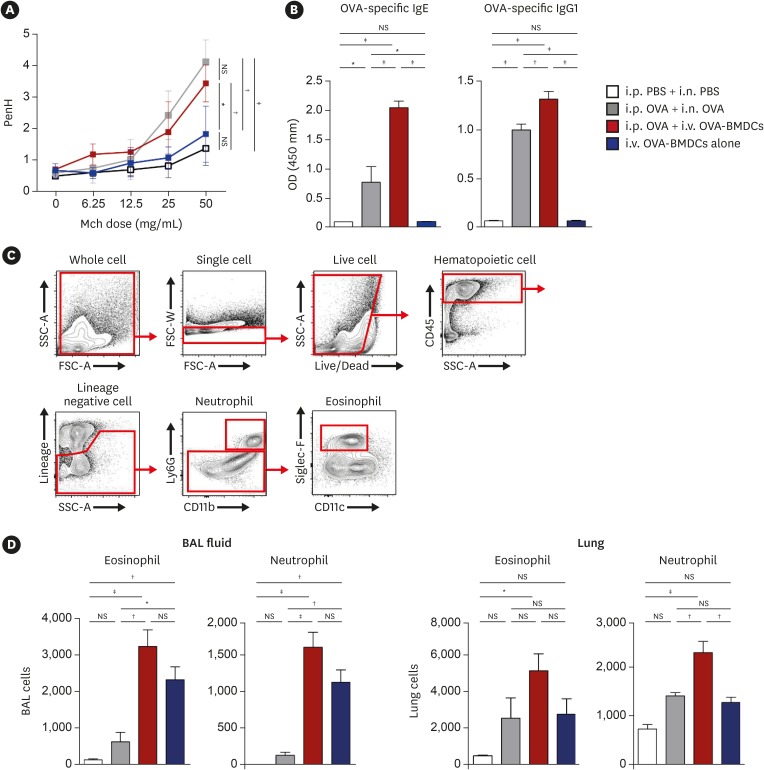
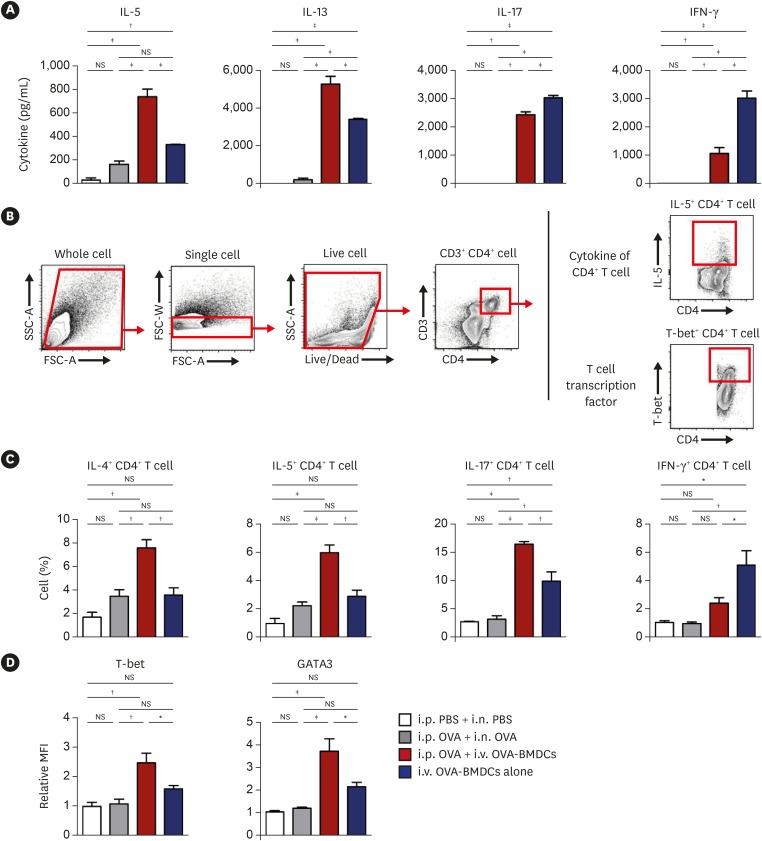
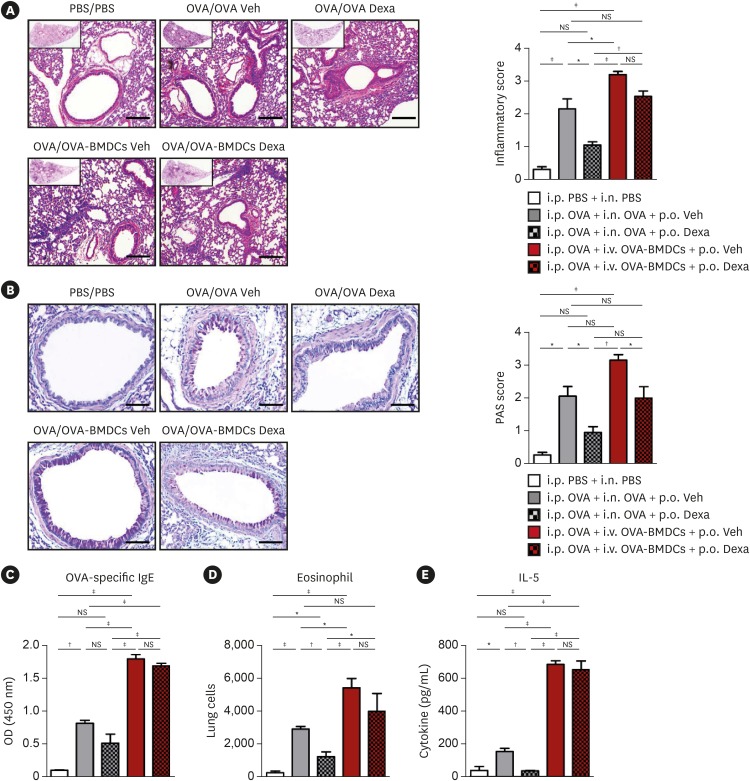
OVA-BMDC-transferred mice developed severe asthmatic immune responses when compared with mice receiving conventional OVA challenge intranasally. Notably, remarkable increases in systemic immunoglobulin (Ig) E and IgG1 responses, Th2/Th17-associated cytokines (interleukin [IL]-5, IL-13 and IL-17), Th2/Th17-skewed T-cell responses, and cellular components, including eosinophils, neutrophils, and goblet cells, were observed in the lungs of OVA-BMDC-transferred mice. Moreover, the asthmatic immune responses and severity of inflammation were correlated with the number of OVA-BMDCs transferred, indicating that the disease severity and asthma type may be adjusted according to the experimental purpose by this method. Furthermore, this model exhibited less variation among the test individuals than the conventional model. In addition, this DCs-based asthma model was partially resistant to steroid treatment.
CONCLUSIONS
A reliable murine model of asthma by intravenous (i.v.) transfer of OVA-BMDCs was successfully established without anesthesia. This model more accurately reflects heterogeneous human asthma, exhibiting a robust Th2/Th17-skewed response and eosinophilic/neutrophilic infiltration with good reproducibility and low variation among individuals. This model will be useful for understanding the pathogenesis of asthma and would serve as an alternative tool for immunological studies on the function of DCs, T-cell responses and new drugs.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Understanding the Mouse Model of Respiratory Allergic Diseases
Sang Chul Park
Korean J Otorhinolaryngol-Head Neck Surg. 2022;65(6):309-318. doi: 10.3342/kjorl-hns.2022.00381.
Reference
-
1. Kim MA, Shin YS, Pham le D, Park HS. Adult asthma biomarkers. Curr Opin Allergy Clin Immunol. 2014; 14:49–54. PMID: 24300416.
Article2. Foster PS, Maltby S, Rosenberg HF, Tay HL, Hogan SP, Collison AM, et al. Modeling TH 2 responses and airway inflammation to understand fundamental mechanisms regulating the pathogenesis of asthma. Immunol Rev. 2017; 278:20–40. PMID: 28658543.3. Kim SH, Uuganbayar U, Trinh HKT, Pham DL, Kim N, Kim M, et al. Evaluation of neutrophil activation status according to the phenotypes of adult asthma. Allergy Asthma Immunol Res. 2019; 11:381–393. PMID: 30912327.
Article4. Liu W, Liu S, Verma M, Zafar I, Good JT, Rollins D, et al. Mechanism of TH2/TH17-predominant and neutrophilic TH2/TH17-low subtypes of asthma. J Allergy Clin Immunol. 2017; 139:1548–1558.e4. PMID: 27702673.5. Kim YM, Kim YS, Jeon SG, Kim YK. Immunopathogenesis of allergic asthma: more than the Th2 hypothesis. Allergy Asthma Immunol Res. 2013; 5:189–196. PMID: 23814671.
Article6. GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017; 5:691–706. PMID: 28822787.7. Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012; 18:673–683. PMID: 22561831.
Article8. Fajt ML, Wenzel SE. Development of new therapies for severe asthma. Allergy Asthma Immunol Res. 2017; 9:3–14. PMID: 27826957.
Article9. Hall S, Agrawal DK. Key mediators in the immunopathogenesis of allergic asthma. Int Immunopharmacol. 2014; 23:316–329. PMID: 24933589.
Article10. Shin YS, Takeda K, Gelfand EW. Understanding asthma using animal models. Allergy Asthma Immunol Res. 2009; 1:10–18. PMID: 20224665.
Article11. Corazza N, Kaufmann T. Novel insights into mechanisms of food allergy and allergic airway inflammation using experimental mouse models. Allergy. 2012; 67:1483–1490. PMID: 23106364.
Article12. Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005; 6:1047–1053. PMID: 16142237.
Article13. Olmez D, Babayigit A, Erbil G, Karaman O, Bagriyanik A, Yilmaz O, et al. Histopathologic changes in two mouse models of asthma. J Investig Allergol Clin Immunol. 2009; 19:132–138.14. Shim JU, Lee SE, Hwang W, Lee C, Park JW, Sohn JH, et al. Flagellin suppresses experimental asthma by generating regulatory dendritic cells and T cells. J Allergy Clin Immunol. 2016; 137:426–435. PMID: 26303344.
Article15. Blyth DI, Wharton TF, Pedrick MS, Savage TJ, Sanjar S. Airway subepithelial fibrosis in a murine model of atopic asthma: suppression by dexamethasone or anti-interleukin-5 antibody. Am J Respir Cell Mol Biol. 2000; 23:241–246. PMID: 10919992.16. Yoshino S, Mizutani N, Matsuoka D, Sae-Wong C. Intratracheal exposure to Fab fragments of an allergen-specific monoclonal antibody regulates asthmatic responses in mice. Immunology. 2014; 141:617–627. PMID: 24303921.
Article17. Casaro M, Souza VR, Oliveira FA, Ferreira CM. OVA-induced allergic airway inflammation mouse model. Methods Mol Biol. 2019; 1916:297–301. PMID: 30535706.
Article18. Liu JN, Suh DH, Trinh HK, Chwae YJ, Park HS, Shin YS. The role of autophagy in allergic inflammation: a new target for severe asthma. Exp Mol Med. 2016; 48:e243. PMID: 27364893.
Article19. Lee T, Kwon HS, Bang BR, Lee YS, Park MY, Moon KA, et al. Grape seed proanthocyanidin extract attenuates allergic inflammation in murine models of asthma. J Clin Immunol. 2012; 32:1292–1304. PMID: 22836658.
Article20. Zakeri A, Russo M. Dual role of toll-like receptors in human and experimental asthma models. Front Immunol. 2018; 9:1027. PMID: 29867994.
Article21. Maltby S, Tay HL, Yang M, Foster PS. Mouse models of severe asthma: Understanding the mechanisms of steroid resistance, tissue remodelling and disease exacerbation. Respirology. 2017; 22:874–885. PMID: 28401621.
Article22. Gaurav R, Agrawal DK. Clinical view on the importance of dendritic cells in asthma. Expert Rev Clin Immunol. 2013; 9:899–919. PMID: 24128155.
Article23. Shin YS, Takeda K, Shiraishi Y, Jeong YY, Domenico J, Jia Y, et al. Microbial heat shock protein 65 attenuates airway hyperresponsiveness and inflammation by modulating the function of dendritic cells. J Immunol. 2012; 189:3404–3410. PMID: 22933632.
Article24. Vroman H, van den Blink B, Kool M. Mode of dendritic cell activation: the decisive hand in Th2/Th17 cell differentiation. Implications in asthma severity? Immunobiology. 2015; 220:254–261. PMID: 25245013.
Article25. de Aragão-França LS, Aragão-França LS, Rocha VCJ, Rocha VCJ, Cronemberger-Andrade A, da Costa FHB, et al. Tolerogenic dendritic cells reduce airway inflammation in a model of dust mite triggered allergic inflammation. Allergy Asthma Immunol Res. 2018; 10:406–419. PMID: 29949837.
Article26. Kim JS, Kim WS, Choi HG, Jang B, Lee K, Park JH, et al. Mycobacterium tuberculosis RpfB drives Th1-type T cell immunity via a TLR4-dependent activation of dendritic cells. J Leukoc Biol. 2013; 94:733–749. PMID: 23825389.
Article27. Yoshioka M, Sagara H, Takahashi F, Harada N, Nishio K, Mori A, et al. Role of multidrug resistance-associated protein 1 in the pathogenesis of allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2009; 296:L30–6. PMID: 18931056.
Article28. Rajavelu P, Chen G, Xu Y, Kitzmiller JA, Korfhagen TR, Whitsett JA. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J Clin Invest. 2015; 125:2021–2031. PMID: 25866971.
Article29. Kamijo S, Takeda H, Tokura T, Suzuki M, Inui K, Hara M, et al. IL-33-mediated innate response and adaptive immune cells contribute to maximum responses of protease allergen-induced allergic airway inflammation. J Immunol. 2013; 190:4489–4499. PMID: 23547117.
Article30. Graffi SJ, Dekan G, Stingl G, Epstein MM. Systemic administration of antigen-pulsed dendritic cells induces experimental allergic asthma in mice upon aerosol antigen rechallenge. Clin Immunol. 2002; 103:176–184. PMID: 12027423.
Article31. Besnard AG, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol. 2011; 41:1675–1686. PMID: 21469105.
Article32. Zhang Y, Zhou X, Zhou B. DC-derived TSLP promotes Th2 polarization in LPS-primed allergic airway inflammation. Eur J Immunol. 2012; 42:1735–1743. PMID: 22585305.
Article33. Lambrecht BN, De Veerman M, Coyle AJ, Gutierrez-Ramos JC, Thielemans K, Pauwels RA. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest. 2000; 106:551–559. PMID: 10953030.
Article34. van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005; 201:981–991. PMID: 15781587.35. Hirose K, Iwata A, Tamachi T, Nakajima H. Allergic airway inflammation: key players beyond the Th2 cell pathway. Immunol Rev. 2017; 278:145–161. PMID: 28658544.
Article36. Raundhal M, Morse C, Khare A, Oriss TB, Milosevic J, Trudeau J, et al. High IFN-γ and low SLPI mark severe asthma in mice and humans. J Clin Invest. 2015; 125:3037–3050. PMID: 26121748.
Article37. Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015; 7:301ra129.
Article38. Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014; 133:1557–1563.e5. PMID: 24332216.
Article39. Sun B, Zhu L, Tao Y, Sun HX, Li Y, Wang P, et al. Characterization and allergic role of IL-33-induced neutrophil polarization. Cell Mol Immunol. 2018; 15:782–793. PMID: 29503441.
Article40. Diver S, Russell RJ, Brightling CE. New and emerging drug treatments for severe asthma. Clin Exp Allergy. 2018; 48:241–252. PMID: 29315966.
Article41. Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014; 134:1175–1186.e7. PMID: 25042748.42. Webb LM, Lundie RJ, Borger JG, Brown SL, Connor LM, Cartwright AN, et al. Type I interferon is required for T helper (Th) 2 induction by dendritic cells. EMBO J. 2017; 36:2404–2418. PMID: 28716804.
Article43. Morita H, Arae K, Unno H, Toyama S, Motomura K, Matsuda A, et al. IL-25 and IL-33 contribute to development of eosinophilic airway inflammation in epicutaneously antigen-sensitized mice. PLoS One. 2015; 10:e0134226. PMID: 26230091.
Article44. Adkinson NF, Bochner BS, Burks AW, Busse WW, Holgate ST, Lemanske RF, et al. Middleton's allergy principles and practice. 8th ed. Philadelphia (PA): Elsevier Saunders;2013.45. Medoff BD, Seung E, Hong S, Thomas SY, Sandall BP, Duffield JS, et al. CD11b+ myeloid cells are the key mediators of Th2 cell homing into the airway in allergic inflammation. J Immunol. 2009; 182:623–635. PMID: 19109196.46. Li BW, de Bruijn MJ, Tindemans I, Lukkes M, KleinJan A, Hoogsteden HC, et al. T cells are necessary for ILC2 activation in house dust mite-induced allergic airway inflammation in mice. Eur J Immunol. 2016; 46:1392–1403. PMID: 27062360.
Article47. Eggert AA, Schreurs MW, Boerman OC, Oyen WJ, de Boer AJ, Punt CJ, et al. Biodistribution and vaccine efficiency of murine dendritic cells are dependent on the route of administration. Cancer Res. 1999; 59:3340–3345. PMID: 10416590.