Korean J Physiol Pharmacol.
2020 Jan;24(1):69-79. 10.4196/kjpp.2020.24.1.69.
Quercetin-induced apoptosis ameliorates vascular smooth muscle cell senescence through AMP-activated protein kinase signaling pathway
- Affiliations
-
- 1Department of Pharmacology, Yeungnam University College of Medicine, Daegu 42415, Korea. hcchoi@med.yu.ac.kr
- 2Department of Biochemistry and Molecular Biology, Yeungnam University College of Medicine, Daegu 42415, Korea.
- 3Smart-aging Convergence Research Center, Yeungnam University College of Medicine, Daegu 42415, Korea.
- KMID: 2466571
- DOI: http://doi.org/10.4196/kjpp.2020.24.1.69
Abstract
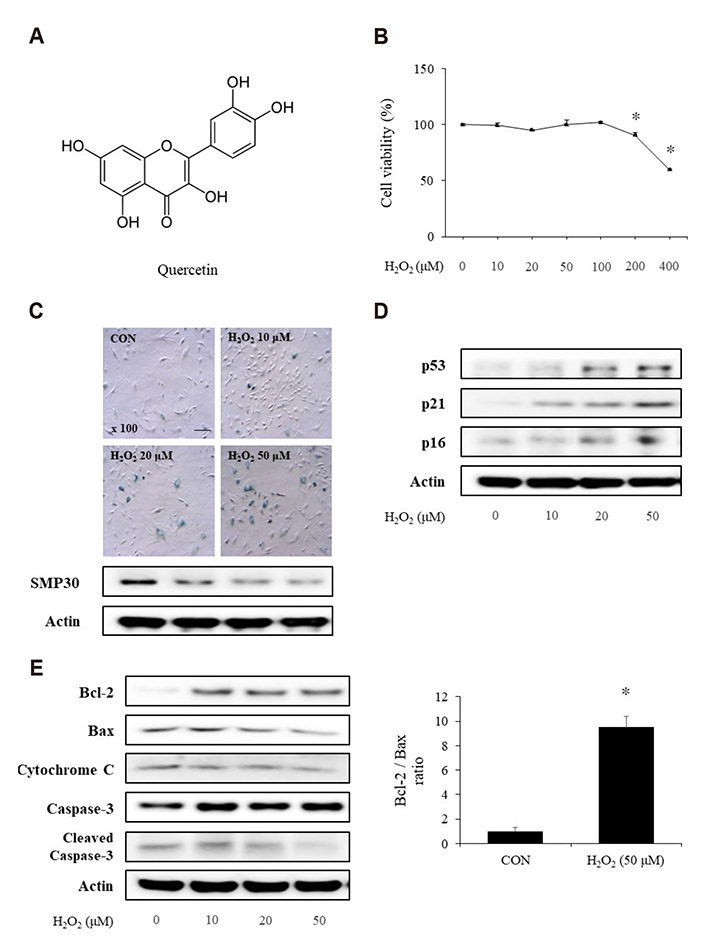
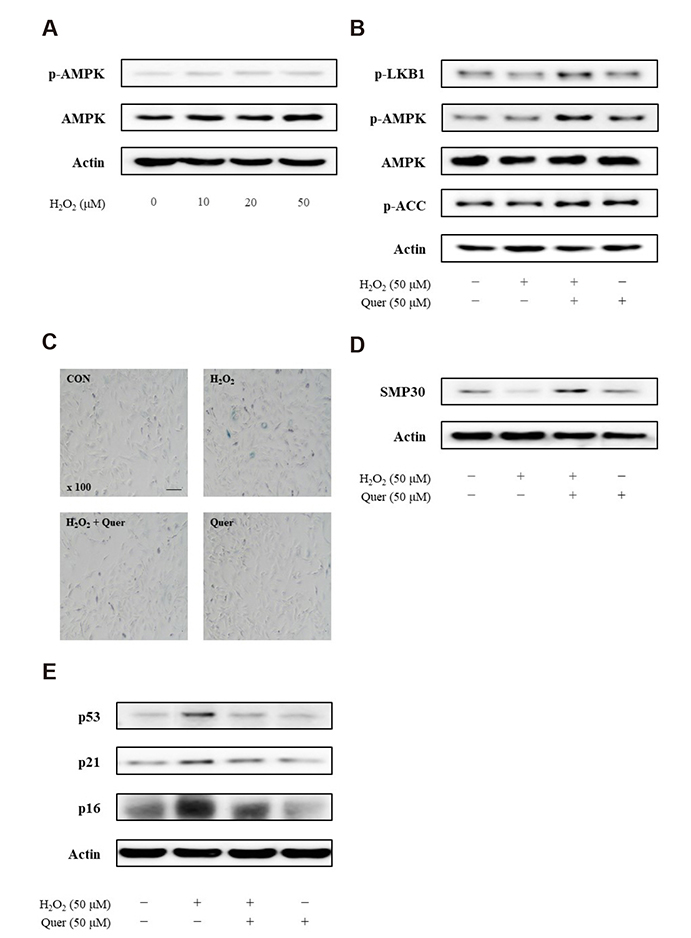
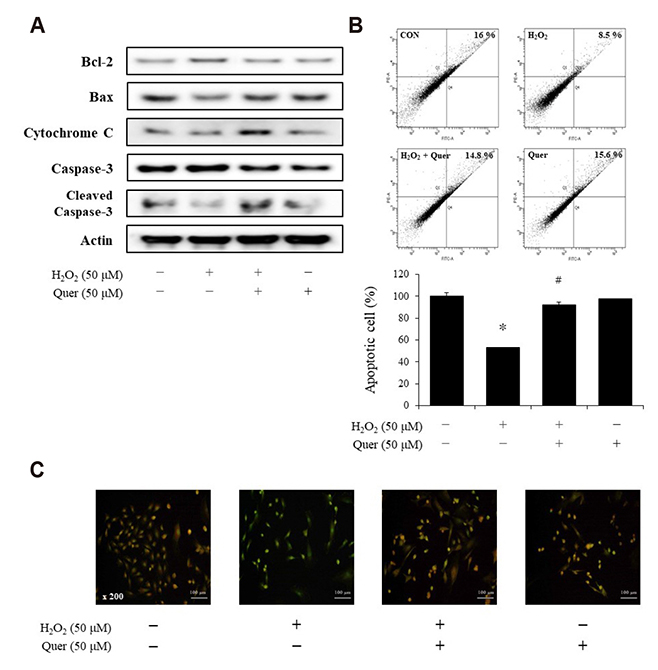
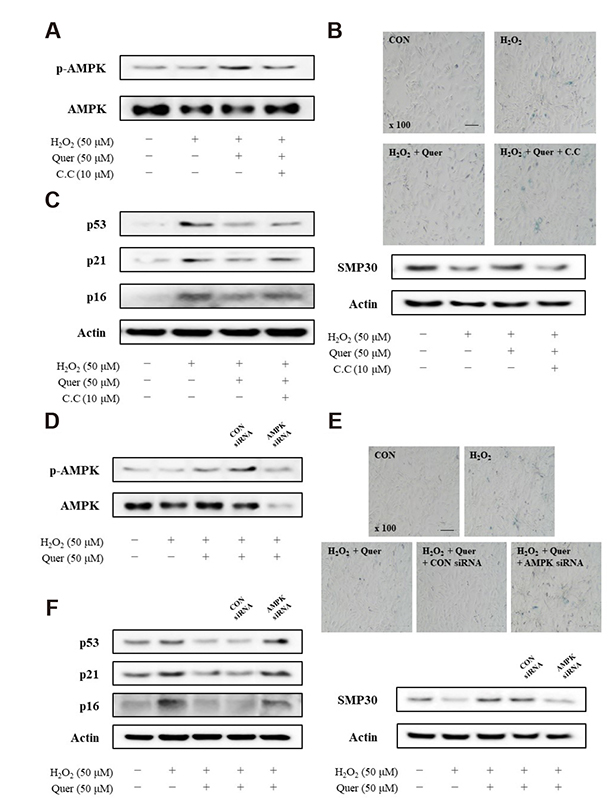
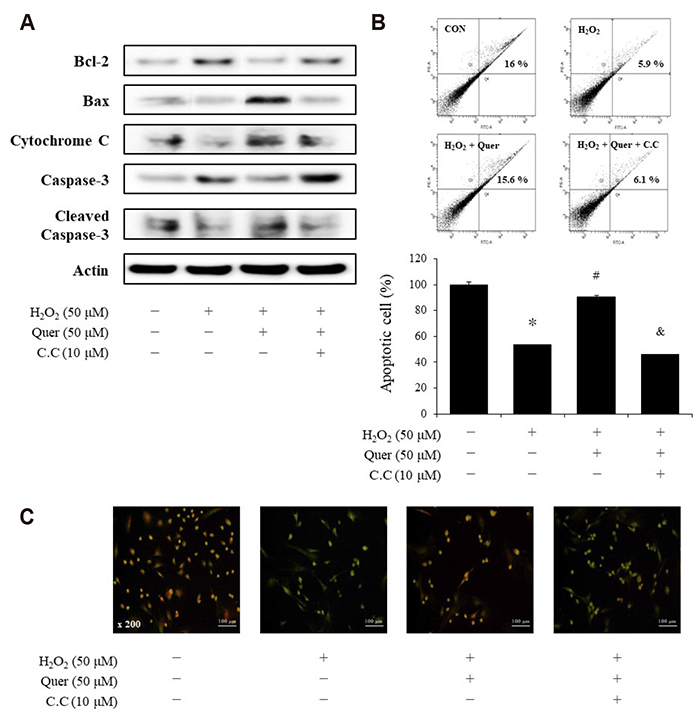
- Aging is one of the risk factors for the development of cardiovascular diseases. During the progression of cellular senescence, cells enter a state of irreversible growth arrest and display resistance to apoptosis. As a flavonoid, quercetin induces apoptosis in various cells. Accordingly, we investigated the relationship between quercetin-induced apoptosis and the inhibition of cellular senescence, and determined the mechanism of oxidative stress-induced vascular smooth muscle cell (VSMC) senescence. In cultured VSMCs, hydrogen peroxide (H₂O₂) dose-dependently induced senescence, which was associated with increased numbers of senescence-associated β-galactosidase-positive cells, decreased expression of SMP30, and activation of p53-p21 and p16 pathways. Along with senescence, expression of the anti-apoptotic protein Bcl-2 was observed to increase and the levels of proteins related to the apoptosis pathway were observed to decrease. Quercetin induced apoptosis through the activation of AMP-activated protein kinase. This action led to the alleviation of oxidative stress-induced VSMC senescence. Furthermore, the inhibition of AMPK activation with compound C and siRNA inhibited apoptosis and aggravated VSMC senescence by reversing p53-p21 and p16 pathways. These results suggest that senescent VSMCs are resistant to apoptosis and quercetin-induced apoptosis attenuated the oxidative stress-induced senescence through activation of AMPK. Therefore, induction of apoptosis by polyphenols such as quercetin may be worthy of attention for its anti-aging effects.
MeSH Terms
Figure
Cited by 1 articles
-
Regulation of Cellular Senescence in Type 2 Diabetes Mellitus: From Mechanisms to Clinical Applications
Kanako Iwasaki, Cristian Abarca, Cristina Aguayo-Mazzucato
Diabetes Metab J. 2023;47(4):441-453. doi: 10.4093/dmj.2022.0416.
Reference
-
1. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003; 107:139–146.2. von Zglinicki T, Petrie J, Kirkwood TB. Telomere-driven replicative senescence is a stress response. Nat Biotechnol. 2003; 21:229–230.
Article3. Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol. 2000; 35:927–945.
Article4. Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995; 92:9363–9367.
Article5. Salminen A, Ojala J, Kaarniranta K. Apoptosis and aging: increased resistance to apoptosis enhances the aging process. Cell Mol Life Sci. 2011; 68:1021–1031.
Article6. Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999; 402:309–313.
Article7. Wang E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res. 1995; 55:2284–2292.8. Chaturvedi V, Qin JZ, Stennett L, Choubey D, Nickoloff BJ. Resistance to UV-induced apoptosis in human keratinocytes during accelerated senescence is associated with functional inactivation of p53. J Cell Physiol. 2004; 198:100–109.
Article9. Ryu SJ, Oh YS, Park SC. Failure of stress-induced downregulation of Bcl-2 contributes to apoptosis resistance in senescent human diploid fibroblasts. Cell Death Differ. 2007; 14:1020–1028.
Article10. Grahame Hardie D. AMP-activated protein kinase: a key regulator of energy balance with many roles in human disease. J Intern Med. 2014; 276:543–559.
Article11. Apfeld J, O'Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004; 18:3004–3009.
Article12. Stenesen D, Suh JM, Seo J, Yu K, Lee KS, Kim JS, Min KJ, Graff JM. Adenosine nucleotide biosynthesis and AMPK regulate adult life span and mediate the longevity benefit of caloric restriction in flies. Cell Metab. 2013; 17:101–112.
Article13. Lakshmanan AP, Watanabe K, Thandavarayan RA, Sari FR, Meilei H, Soetikno V, Arumugam S, Giridharan VV, Suzuki K, Kodama M. Curcumin attenuates hyperglycaemia-mediated AMPK activation and oxidative stress in cerebrum of streptozotocin-induced diabetic rat. Free Radic Res. 2011; 45:788–795.
Article14. Lee KY, Kim JR, Choi HC. Genistein-induced LKB1-AMPK activation inhibits senescence of VSMC through autophagy induction. Vascul Pharmacol. 2016; 81:75–82.
Article15. Peterson JJ, Dwyer JT, Jacques PF, McCullough ML. Associations between flavonoids and cardiovascular disease incidence or mortality in European and US populations. Nutr Rev. 2012; 70:491–508.
Article16. Russo GL. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem Pharmacol. 2007; 74:533–544.
Article17. Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol. 1995; 33:1061–1080.
Article18. Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008; 269:315–325.
Article19. Nguyen LT, Lee YH, Sharma AR, Park JB, Jagga S, Sharma G, Lee SS, Nam JS. Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity. Korean J Physiol Pharmacol. 2017; 21:205–213.
Article20. Kim GT, Lee SH, Kim YM. Quercetin regulates sestrin 2-AMPK-mTOR signaling pathway and induces apoptosis via increased intracellular ROS in HCT116 colon cancer cells. J Cancer Prev. 2013; 18:264–270.
Article21. Lee YK, Hwang JT, Kwon DY, Surh YJ, Park OJ. Induction of apoptosis by quercetin is mediated through AMPKalpha1/ASK1/p38 pathway. Cancer Lett. 2010; 292:228–236.22. Su Q, Peng M, Zhang Y, Xu W, Darko KO, Tao T, Huang Y, Tao X, Yang X. Quercetin induces bladder cancer cells apoptosis by activation of AMPK signaling pathway. Am J Cancer Res. 2016; 6:498–508.23. Perez-Vizcaino F, Bishop-Bailley D, Lodi F, Duarte J, Cogolludo A, Moreno L, Bosca L, Mitchell JA, Warner TD. The flavonoid quercetin induces apoptosis and inhibits JNK activation in intimal vascular smooth muscle cells. Biochem Biophys Res Commun. 2006; 346:919–925.
Article24. Ido Y, Duranton A, Lan F, Cacicedo JM, Chen TC, Breton L, Ruderman NB. Acute activation of AMP-activated protein kinase prevents H2O2-induced premature senescence in primary human keratinocytes. PLoS One. 2012; 7:e35092.25. Pu X, Yu S, Fan W, Liu L, Ma X, Ren J. Guiqi polysaccharide protects the normal human fetal lung fibroblast WI-38 cells from H2O2-induced premature senescence. Int J Clin Exp Pathol. 2015; 8:4398–4407.26. Sheng L, Jiao B, Shao L, Bi S, Cheng C, Zhang J, Jiang Y. Probucol inhibits hydrogen peroxide to induce apoptosis of vascular smooth muscle cells. Mol Med Rep. 2013; 7:1185–1190.
Article27. Yang H, Xie Y, Yang D, Ren D. Oxidative stress-induced apoptosis in granulosa cells involves JNK, p53 and Puma. Oncotarget. 2017; 8:25310–25322.
Article28. Kim SG, Kim JR, Choi HC. Quercetin-induced AMP-activated protein kinase activation attenuates vasoconstriction through LKB1-AMPK signaling pathway. J Med Food. 2018; 21:146–153.
Article29. Bennett MR, Macdonald K, Chan SW, Boyle JJ, Weissberg PL. Cooperative interactions between RB and p53 regulate cell proliferation, cell senescence, and apoptosis in human vascular smooth muscle cells from atherosclerotic plaques. Circ Res. 1998; 82:704–712.
Article30. Mercer J, Mahmoudi M, Bennett M. DNA damage, p53, apoptosis and vascular disease. Mutat Res. 2007; 621:75–86.
Article31. Ido Y, Duranton A, Lan F, Weikel KA, Breton L, Ruderman NB. Resveratrol prevents oxidative stress-induced senescence and proliferative dysfunction by activating the AMPK-FOXO3 cascade in cultured primary human keratinocytes. PLoS One. 2015; 10:e0115341.
Article32. Han X, Tai H, Wang X, Wang Z, Zhou J, Wei X, Ding Y, Gong H, Mo C, Zhang J, Qin J, Ma Y, Huang N, Xiang R, Xiao H. AMPK activation protects cells from oxidative stress-induced senescence via autophagic flux restoration and intracellular NAD+ elevation. Aging Cell. 2016; 15:416–427.33. Xiao N, Mei F, Sun Y, Pan G, Liu B, Liu K. Quercetin, luteolin, and epigallocatechin gallate promote glucose disposal in adipocytes with regulation of AMP-activated kinase and/or sirtuin 1 activity. Planta Med. 2014; 80:993–1000.
Article34. Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O'Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015; 14:644–658.
Article35. Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD, Kirkland JL. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016; 15:428–435.
Article36. Pu Y, Zhang H, Wang P, Zhao Y, Li Q, Wei X, Cui Y, Sun J, Shang Q, Liu D, Zhu Z. Dietary curcumin ameliorates aging-related cerebrovascular dysfunction through the AMPK/uncoupling protein 2 pathway. Cell Physiol Biochem. 2013; 32:1167–1177.
Article37. Feresin RG, Huang J, Klarich DS, Zhao Y, Pourafshar S, Arjmandi BH, Salazar G. Blackberry, raspberry and black raspberry polyphenol extracts attenuate angiotensin II-induced senescence in vascular smooth muscle cells. Food Funct. 2016; 7:4175–4187.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- AMP kinase/cyclooxygenase-2 pathway regulates proliferation and apoptosis of cancer cells treated with quercetin
- Quercetin induces cell death by caspase-dependent and p38 MAPK pathway in EGFR mutant lung cancer cells
- Losartan Inhibits Vascular Smooth Muscle Cell Proliferation through Activation of AMP-Activated Protein Kinase
- Anti-malarial Drugs Reduce Vascular Smooth Muscle Cell Proliferation via Activation of AMPK and Inhibition of Smad3 Signaling
- 5-aminoimidazole-4-carboxamide Riboside Induces Apoptosis Through AMP-activated Protein Kinase-independent and NADPH Oxidase-dependent Pathways