Allergy Asthma Immunol Res.
2020 Jan;12(1):56-71. 10.4168/aair.2020.12.1.56.
Particulate Matter 2.5 Causes Deficiency in Barrier Integrity in Human Nasal Epithelial Cells
- Affiliations
-
- 1Department of Otolaryngology Head and Neck Surgery, Beijing TongRen Hospital, Capital Medical University, Beijing, China. dr.luozhang@139.com, wangcs830@126.com
- 2Beijing Key Laboratory of Nasal Diseases, Beijing Institute of Otolaryngology, Beijing, China.
- 3Swiss Institute of Allergy and Asthma Research (SIAF), University of Zurich, Davos, Switzerland.
- 4Christine Kühne-Center for Allergy Research and Education, Davos, Switzerland.
- KMID: 2462572
- DOI: http://doi.org/10.4168/aair.2020.12.1.56
Abstract
- PURPOSE
The effect of air pollution-related particulate matter (PM) on epithelial barrier function and tight junction (TJ) expression in human nasal mucosa has not been studied to date. This study therefore aimed to assess the direct impact of PM with an aerodynamic diameter less than 2.5 μm (PM2.5) on the barrier function and TJ molecular expression of human nasal epithelial cells.
METHODS
Air-liquid interface cultures were established with epithelial cells derived from noninflammatory nasal mucosal tissue collected from patients undergoing paranasal sinus surgery. Confluent cultures were exposed to 50 or 100 µg/mL PM2.5 for up to 72 hours, and assessed for 1) epithelial barrier integrity as measured by transepithelial resistance (TER) and permeability of fluorescein isothiocyanate (FITC) 4 kDa; 2) expression of TJs using real-time quantitative polymerase chain reaction and immunofluorescence staining, and 3) proinflammatory cytokines by luminometric bead array or enzyme-linked immunosorbent assay.
RESULTS
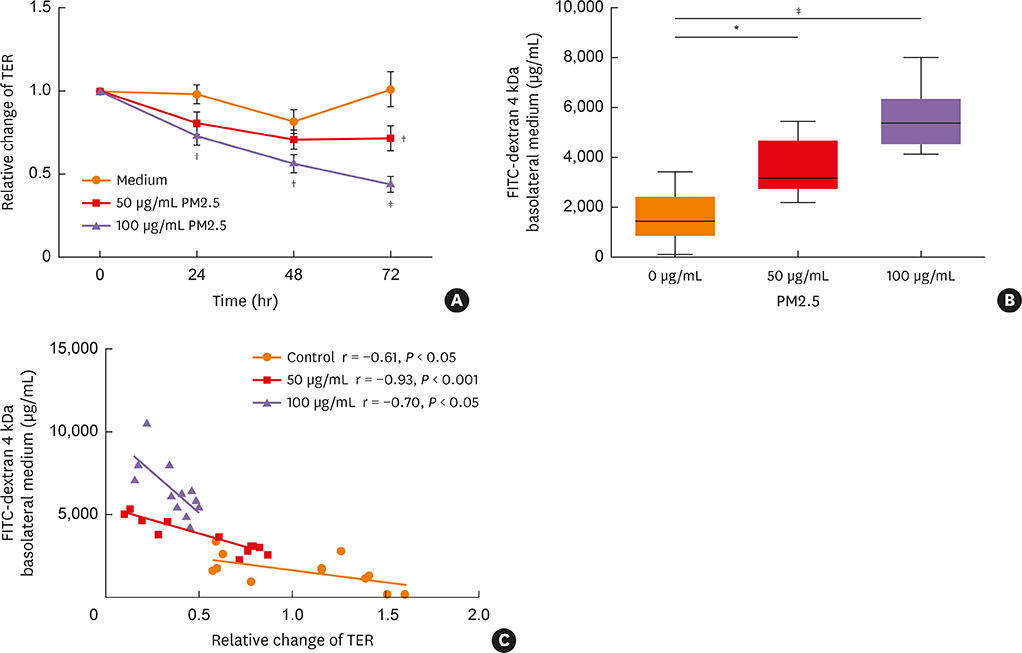
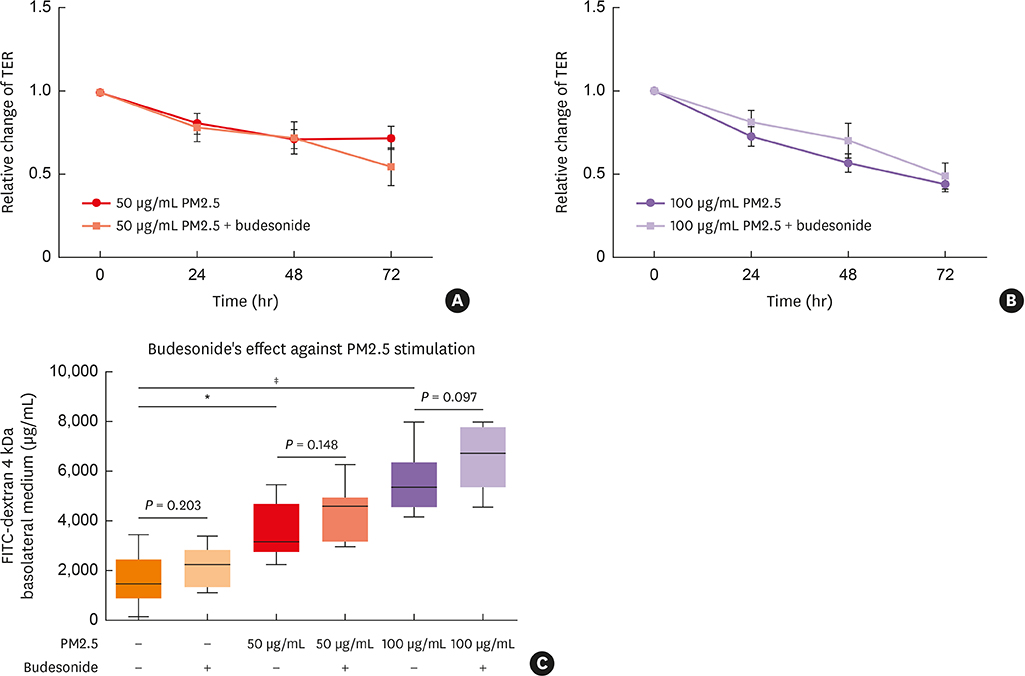
Compared to control medium, 50 and/or 100 µg/mL PM2.5-treatment 1) significantly decreased TER and increased FITC permeability, which could not be restored by budesonide pretreatment; 2) significantly decreased the expression of claudin-1 messenger RNA, claudin-1, occludin and ZO-1 protein; and 3) significantly increased production of the cytokines interleukin-8, TIMP metallopeptidase inhibitor 1 and thymic stromal lymphopoietin.
CONCLUSIONS
Exposure to PM2.5 may lead to loss of barrier function in human nasal epithelium through decreased expression of TJ proteins and increased release of proinflammatory cytokines. These results suggest an important mechanism of susceptibility to rhinitis and rhinosinusitis in highly PM2.5-polluted areas.
Keyword
MeSH Terms
-
Asthma
Budesonide
Claudin-1
Cytokines
Enzyme-Linked Immunosorbent Assay
Epithelial Cells*
Fluorescein
Fluorescein-5-isothiocyanate
Fluorescent Antibody Technique
Humans*
Interleukin-8
Mucous Membrane
Nasal Mucosa
Occludin
Particulate Matter*
Permeability
Polymerase Chain Reaction
Rhinitis
RNA, Messenger
Tight Junctions
Budesonide
Claudin-1
Cytokines
Fluorescein
Fluorescein-5-isothiocyanate
Interleukin-8
Occludin
Particulate Matter
RNA, Messenger
Figure
Cited by 1 articles
-
Epithelial-Mesenchymal Transition in Chronic Rhinosinusitis
Taewoong Choi, Simyoung Ryu, Jun-Sang Bae, Shin Hyuk Yoo, Ji-Hun Mo
J Rhinol. 2024;31(2):67-77. doi: 10.18787/jr.2024.00022.
Reference
-
1. Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014; 134:509–520.
Article2. Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-γ and IL-4. J Allergy Clin Immunol. 2012; 130:1087–1096.e10.
Article3. Steelant B, Farré R, Wawrzyniak P, Belmans J, Dekimpe E, Vanheel H, et al. Impaired barrier function in patients with house dust mite-induced allergic rhinitis is accompanied by decreased occludin and zonula occludens-1 expression. J Allergy Clin Immunol. 2016; 137:1043–1053.e5.
Article4. Aghapour M, Raee P, Moghaddam SJ, Hiemstra PS, Heijink IH. Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: role of cigarette smoke exposure. Am J Respir Cell Mol Biol. 2018; 58:157–169.
Article5. Holgate ST. Mechanisms of asthma and implications for its prevention and treatment: a personal journey. Allergy Asthma Immunol Res. 2013; 5:343–347.
Article6. Darrow LA, Klein M, Flanders WD, Mulholland JA, Tolbert PE, Strickland MJ. Air pollution and acute respiratory infections among children 0–4 years of age: an 18-year time-series study. Am J Epidemiol. 2014; 180:968–977.
Article7. Feng C, Li J, Sun W, Zhang Y, Wang Q. Impact of ambient fine particulate matter (PM2.5) exposure on the risk of influenza-like-illness: a time-series analysis in Beijing, China. Environ Health. 2016; 15:17.
Article8. Bernstein DI. Diesel exhaust exposure, wheezing and sneezing. Allergy Asthma Immunol Res. 2012; 4:178–183.
Article9. Kumar RK, Shadie AM, Bucknall MP, Rutlidge H, Garthwaite L, Herbert C, et al. Differential injurious effects of ambient and traffic-derived particulate matter on airway epithelial cells. Respirology. 2015; 20:73–79.
Article10. Golebski K, Röschmann KI, Toppila-Salmi S, Hammad H, Lambrecht BN, Renkonen R, et al. The multi-faceted role of allergen exposure to the local airway mucosa. Allergy. 2013; 68:152–160.
Article11. Sekiyama A, Gon Y, Terakado M, Takeshita I, Kozu Y, Maruoka S, et al. Glucocorticoids enhance airway epithelial barrier integrity. Int Immunopharmacol. 2012; 12:350–357.
Article12. Fujita H, Chalubinski M, Rhyner C, Indermitte P, Meyer N, Ferstl R, et al. Claudin-1 expression in airway smooth muscle exacerbates airway remodeling in asthmatic subjects. J Allergy Clin Immunol. 2011; 127:1612–1621.e8.
Article13. Lee JW, Hsiao WT, Chen HY, Hsu LP, Chen PR, Lin MD, et al. Upregulated claudin-1 expression confers resistance to cell death of nasopharyngeal carcinoma cells. Int J Cancer. 2010; 126:1353–1366.
Article14. Nair A, Vaidyanathan S, Clearie K, Williamson P, Meldrum K, Lipworth BJ. Steroid sparing effects of intranasal corticosteroids in asthma and allergic rhinitis. Allergy. 2010; 65:359–367.
Article15. Derendorf H, Meltzer EO. Molecular and clinical pharmacology of intranasal corticosteroids: clinical and therapeutic implications. Allergy. 2008; 63:1292–1300.
Article16. Zhang Y, Lou H, Wang Y, Li Y, Zhang L, Wang C. Comparison of corticosteroids by 3 approaches to the treatment of chronic rhinosinusitis with nasal polyps. Allergy Asthma Immunol Res. 2019; 11:482–497.
Article17. Koizumi J, Kojima T, Kamekura R, Kurose M, Harimaya A, Murata M, et al. Changes of gap and tight junctions during differentiation of human nasal epithelial cells using primary human nasal epithelial cells and primary human nasal fibroblast cells in a noncontact coculture system. J Membr Biol. 2007; 218:1–7.
Article18. Ohkuni T, Kojima T, Ogasawara N, Masaki T, Ninomiya T, Kikuchi S, et al. Expression and localization of tricellulin in human nasal epithelial cells in vivo and in vitro. Med Mol Morphol. 2009; 42:204–211.19. Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010; 5:119–144.
Article20. Pan TL, Wang PW, Aljuffali IA, Huang CT, Lee CW, Fang JY. The impact of urban particulate pollution on skin barrier function and the subsequent drug absorption. J Dermatol Sci. 2015; 78:51–60.
Article21. Oppenheim HA, Lucero J, Guyot AC, Herbert LM, McDonald JD, Mabondzo A, et al. Exposure to vehicle emissions results in altered blood brain barrier permeability and expression of matrix metalloproteinases and tight junction proteins in mice. Part Fibre Toxicol. 2013; 10:62.
Article22. Wang T, Wang L, Moreno-Vinasco L, Lang GD, Siegler JH, Mathew B, et al. Particulate matter air pollution disrupts endothelial cell barrier via calpain-mediated tight junction protein degradation. Part Fibre Toxicol. 2012; 9:35.
Article23. Mutlu EA, Engen PA, Soberanes S, Urich D, Forsyth CB, Nigdelioglu R, et al. Particulate matter air pollution causes oxidant-mediated increase in gut permeability in mice. Part Fibre Toxicol. 2011; 8:19.
Article24. Liang Y, Fang L, Pan H, Zhang K, Kan H, Brook JR, et al. PM2.5 in Beijing - temporal pattern and its association with influenza. Environ Health. 2014; 13:102.
Article25. Kim DW, Chung SK, Na Y. Numerical study on the air conditioning characteristics of the human nasal cavity. Comput Biol Med. 2017; 86:18–30.
Article26. Heijink IH, Jonker MR, de Vries M, van Oosterhout AJ, Telenga E, Ten Hacken NH, et al. Budesonide and fluticasone propionate differentially affect the airway epithelial barrier. Respir Res. 2016; 17:2.
Article27. Fischer A, Gluth M, Weege F, Pape UF, Wiedenmann B, Baumgart DC, et al. Glucocorticoids regulate barrier function and claudin expression in intestinal epithelial cells via MKP-1. Am J Physiol Gastrointest Liver Physiol. 2014; 306:G218–G228.
Article28. Schamberger AC, Mise N, Jia J, Genoyer E, Yildirim AÖ, Meiners S, et al. Cigarette smoke-induced disruption of bronchial epithelial tight junctions is prevented by transforming growth factor-β. Am J Respir Cell Mol Biol. 2014; 50:1040–1052.
Article29. Henriquez OA, Den Beste K, Hoddeson EK, Parkos CA, Nusrat A, Wise SK. House dust mite allergen Der p 1 effects on sinonasal epithelial tight junctions. Int Forum Allergy Rhinol. 2013; 3:630–635.
Article30. Fukuoka A, Matsushita K, Morikawa T, Takano H, Yoshimoto T. Diesel exhaust particles exacerbate allergic rhinitis in mice by disrupting the nasal epithelial barrier. Clin Exp Allergy. 2016; 46:142–152.
Article31. Rogers GA, Den Beste K, Parkos CA, Nusrat A, Delgaudio JM, Wise SK. Epithelial tight junction alterations in nasal polyposis. Int Forum Allergy Rhinol. 2011; 1:50–54.
Article32. Altunbulakli C, Costa R, Lan F, Zhang N, Akdis M, Bachert C, et al. Staphylococcus aureus enhances the tight junction barrier integrity in healthy nasal tissue, but not in nasal polyps. J Allergy Clin Immunol. 2018; 142:665–668.e8.33. Zhang Y, Lan F, Li Y, Wang C, Zhang L. Formation of papillary mucosa folds and enhancement of epithelial barrier in odontogenic sinusitis. Int Forum Allergy Rhinol. 2019.
Article34. Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008; 226:172–190.
Article35. Oboki K, Nakae S, Matsumoto K, Saito H. IL-33 and airway inflammation. Allergy Asthma Immunol Res. 2011; 3:81–88.
Article36. Reynolds CJ, Quigley K, Cheng X, Suresh A, Tahir S, Ahmed-Jushuf F, et al. Lung defense through IL-8 carries a cost of chronic lung remodeling and impaired function. Am J Respir Cell Mol Biol. 2018; 59:557–571.
Article37. Yu H, Huang X, Ma Y, Gao M, Wang O, Gao T, et al. Interleukin-8 regulates endothelial permeability by down-regulation of tight junction but not dependent on integrins induced focal adhesions. Int J Biol Sci. 2013; 9:966–979.
Article38. Vermeer PD, Denker J, Estin M, Moninger TO, Keshavjee S, Karp P, et al. MMP9 modulates tight junction integrity and cell viability in human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2009; 296:L751–62.
Article39. Park CS, Kim TB, Moon KA, Bae YJ, Lee HR, Jang MK, et al. Chlamydophila pneumoniae enhances secretion of VEGF, TGF-beta and TIMP-1 from human bronchial epithelial cells under Th2 dominant microenvironment. Allergy Asthma Immunol Res. 2010; 2:41–47.40. Bleck B, Tse DB, Gordon T, Ahsan MR, Reibman J. Diesel exhaust particle-treated human bronchial epithelial cells upregulate Jagged-1 and OX40 ligand in myeloid dendritic cells via thymic stromal lymphopoietin. J Immunol. 2010; 185:6636–6645.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Particulate Matter Induces NLRP3 Inflammasome-Mediated Pyroptosis in Human Nasal Epithelial Cells
- Epithelial-Mesenchymal Transition in Chronic Rhinosinusitis
- Association of Particulate Matter With ENT Diseases
- Effect of Air Pollutants on Allergic Inflammation in Structural Cells of the Nasal Mucosa
- Particulate Matter 10 from Asian Dust Storms Induces the Expression of Reactive Oxygen Species, NF-kappaB, TGF-beta and Fibronectin in WI-26 VA4 Epithelial Cells