Yonsei Med J.
2019 Nov;60(11):1093-1102. 10.3349/ymj.2019.60.11.1093.
Inhibition of miRNA-222-3p Relieves Staphylococcal Enterotoxin B-Induced Liver Inflammatory Injury by Upregulating Suppressors of Cytokine Signaling 1
- Affiliations
-
- 1Department of Clinical Laboratory, the Third People's Hospital of Dalian, Dalian, China.
- 2Department of Clinical Laboratory, the Baotou Medical College of Inner Mongolia University of Science and Technology, Inner Mongolia, China.
- 3Department of Clinical Laboratory, the Hongqi Hospital Affiliated to Mudanjiang Medical University, Mudanjiang, China.
- 4Department of Clinical Laboratory, the Second Hospital of Dalian Medical University, Dalian, China. zdgkua@163.com
- KMID: 2460227
- DOI: http://doi.org/10.3349/ymj.2019.60.11.1093
Abstract
- PURPOSE
Staphylococcal enterotoxin B (SEB) has been well-documented to induce liver injury. miRNA-222-3p (miR-222-3p) was implicated in SEB-induced lung injury and several liver injuries. This study aimed to explore the role of miR-222-3p in SEB-induced liver injury.
MATERIALS AND METHODS
Expression of miR-222-3p and suppressors of cytokine signaling 1 (SOCS1) was detected using real-time quantitative PCR and western blot. Liver injury was determined by levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and inflammatory cytokines, numbers of infiltrating mononuclear cells using AST/ALT assay kit, enzyme-linked immunosorbent assay (ELISA), and hematoxylin-eosin staining, respectively. Target binding between miR-222-3p and SOCS1 was predicted on targetScan software, and confirmed by luciferase reporter assay.
RESULTS
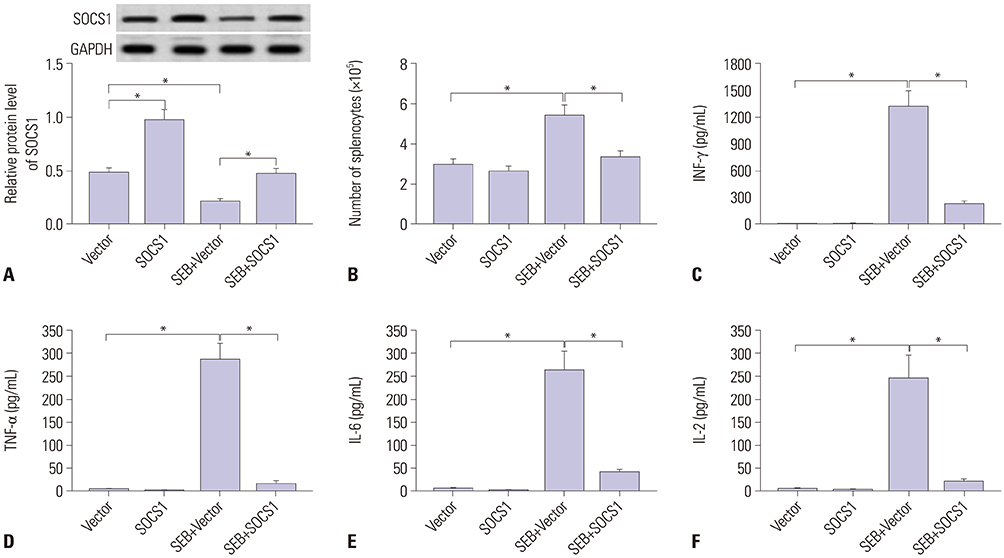
SEB induced liver injury in D-galactosamine (D-gal)-sensitized mice, as demonstrated by increased serum levels of AST and ALT, elevated release of interferon-gamma (INF-γ), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and IL-2, and promoted infiltrating immune cells into liver. Expression of miR-222-3p was dramatically upregulated, and SOCS1 was downregulated in SEB-induced liver injury both in mice and splenocytes. Moreover, miR-222-3p knockout (KO) mice exhibited alleviated liver injury accompanied with SOCS1 upregulation. Besides, splenocytes under SEB challenge released less INF-γ, TNF-α, IL-6, and IL-2 during miR-222-3p knockdown. Mechanically, SOCS1 was targeted and downregulated by miR-222-3p. Upregulation of SOCS1 attenuated INF-γ, TNF-α, IL-6, and IL-2 release in SEB-induced splenocytes; downregulation of SOCS1 could block the suppressive role of miR-222-3p knockdown in SEB-induced splenocytes.
CONCLUSION
Inhibition of miR-222-3p relieves SEB-induced liver inflammatory injury by upregulating SOCS1, thereby providing the first evidence of miR-222-3p in SEB-induced liver injury.
Keyword
MeSH Terms
-
Alanine Transaminase
Animals
Aspartate Aminotransferases
Blotting, Western
Cytokines
Down-Regulation
Enterotoxins*
Enzyme-Linked Immunosorbent Assay
Interferon-gamma
Interleukin-2
Interleukin-6
Liver*
Luciferases
Lung Injury
Mice
Polymerase Chain Reaction
Tumor Necrosis Factor-alpha
Up-Regulation
Alanine Transaminase
Aspartate Aminotransferases
Cytokines
Enterotoxins
Interferon-gamma
Interleukin-2
Interleukin-6
Luciferases
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Pinchuk IV, Beswick EJ, Reyes VE. Staphylococcal enterotoxins. Toxins (Basel). 2010; 2:2177–2197.
Article2. Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009; 7:629–641.
Article3. Henghold WB 2nd. Other biologic toxin bioweapons: ricin, staphylococcal enterotoxin B, and trichothecene mycotoxins. Dermatol Clin. 2004; 22:257–262. v
Article4. Nagaki M, Tanaka M, Sugiyama A, Ohnishi H, Moriwaki H. Interleukin-10 inhibits hepatic injury and tumor necrosis factor-alpha and interferon-gamma mRNA expression induced by staphylococcal enterotoxin B or lipopolysaccharide in galactosamine-sensitized mice. J Hepatol. 1999; 31:815–824.
Article5. Rieder SA, Nagarkatti P, Nagarkatti M. CD1d-independent activation of invariant natural killer T cells by staphylococcal enterotoxin B through major histocompatibility complex class II/T cell receptor interaction results in acute lung injury. Infect Immun. 2011; 79:3141–3148.
Article6. Krakauer T. Staphylococcal superantigens: pyrogenic toxins induce toxic shock. Toxins (Basel). 2019; 11:E178.
Article7. Ler SG, Lee FK, Gopalakrishnakone P. Trends in detection of warfare agents. Detection methods for ricin, staphylococcal enterotoxin B and T-2 toxin. J Chromatogr A. 2006; 1133:1–12.8. Dilda F, Gioia G, Pisani L, Restelli L, Lecchi C, Albonico F, et al. Escherichia coli lipopolysaccharides and Staphylococcus aureus enterotoxin B differentially modulate inflammatory microRNAs in bovine monocytes. Vet J. 2012; 192:514–516.
Article9. Amini S, Abak A, Sakhinia E, Abhari A. MicroRNA-221 and microrna-222 in common human cancers: expression, function, and triggering of tumor progression as a key modulator. Lab Med. 2019; 05. 02. [Epub]. Available at: https://doi.org/10.1093/labmed/lmz002.
Article10. Fiorino S, Bacchi-Reggiani ML, Visani M, Acquaviva G, Fornelli A, Masetti M, et al. MicroRNAs as possible biomarkers for diagnosis and prognosis of hepatitis B- and C-related-hepatocellular-carcinoma. World J Gastroenterol. 2016; 22:3907–3936.
Article11. Mirzaei HR, Sahebkar A, Mohammadi M, Yari R, Salehi H, Jafari MH, et al. Circulating microRNAs in hepatocellular carcinoma: potential diagnostic and prognostic biomarkers. Curr Pharm Des. 2016; 22:5257–5269.
Article12. Qi P, Cheng SQ, Wang H, Li N, Chen YF, Gao CF. Serum microRNAs as biomarkers for hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection. PLoS One. 2011; 6:e28486.
Article13. Motawi TM, Sadik NA, Shaker OG, Ghaleb MH. Elevated serum microRNA-122/222 levels are potential diagnostic biomarkers in Egyptian patients with chronic hepatitis C but not hepatic cancer. Tumour Biol. 2016; 37:9865–9874.
Article14. Higashi M, Yoneda M, Nakagawa T, Ikeda M, Ito T. miR-222 regulates proliferation of primary mouse hepatocytes in vitro. Biochem Biophys Res Commun. 2019; 511:644–649.15. Selten JW, Verhoeven CJ, Heedfeld V, Roest HP, de Jonge J, Pirenne J, et al. The release of microRNA-122 during liver preservation is associated with early allograft dysfunction and graft survival after transplantation. Liver Transpl. 2017; 23:946–956.
Article16. Elliott DM, Nagarkatti M, Nagarkatti PS. 3,39-diindolylmethane ameliorates staphylococcal enterotoxin B-induced acute lung injury through alterations in the expression of microRNA that target apoptosis and cell-cycle arrest in activated T cells. J Pharmacol Exp Ther. 2016; 357:177–187.
Article17. Rao R, Nagarkatti P, Nagarkatti M. Role of miRNA in the regulation of inflammatory genes in staphylococcal enterotoxin B-induced acute inflammatory lung injury and mortality. Toxicol Sci. 2015; 144:284–297.
Article18. Ying J, Qiu X, Lu Y, Zhang M. SOCS1 and its potential clinical role in tumor. Pathol Oncol Res. 2019; 02. 13. [Epub]. Available at: https://doi.org/10.1007/s12253-019-00612-5.
Article19. Inagaki-Ohara K, Kondo T, Ito M, Yoshimura A. SOCS, inflammation, and cancer. JAKSTAT. 2013; 2:e24053.
Article20. Mann M, Mehta A, Zhao JL, Lee K, Marinov GK, Garcia-Flores Y, et al. An NF-κB-microRNA regulatory network tunes macrophage inflammatory responses. Nat Commun. 2017; 8:851.
Article21. Yu J, Zhang W, Qian H, Tang H, Lin W, Lu B. SOCS1 regulates hepatic regenerative response and provides prognostic makers for acute obstructive cholangitis. Sci Rep. 2017; 7:9482.
Article22. Tan L, Jiang W, Lu A, Cai H, Kong L. miR-155 aggravates liver ischemia/ reperfusion injury by suppressing SOCS1 in mice. Transplant Proc. 2018; 50:3831–3839.
Article23. Li SS, Yang M, Chen YP, Tang XY, Zhang SG, Ni SL, et al. Dendritic cells with increased expression of suppressor of cytokine signaling 1(SOCS1) gene ameliorate lipopolysaccharide/d-galactosamineinduced acute liver failure. Mol Immunol. 2018; 101:10–18.
Article24. Araújo Júnior RF, Garcia VB, Leitão RF, Brito GA, Miguel Ede C, Guedes PM, et al. Carvedilol improves inflammatory response, oxidative stress and fibrosis in the alcohol-induced liver injury in rats by regulating kuppfer cells and hepatic stellate cells. PLoS One. 2016; 11:e0148868.
Article25. Rao R, Rieder SA, Nagarkatti P, Nagarkatti M. Staphylococcal enterotoxin B-induced microRNA-155 targets SOCS1 to promote acute inflammatory lung injury. Infect Immun. 2014; 82:2971–2979.
Article26. Plaza R, Vidal S, Rodriguez-Sanchez JL, Juarez C. Implication of STAT1 and STAT3 transcription factors in the response to superantigens. Cytokine. 2004; 25:1–10.
Article27. Kadhim S, Singh NP, Zumbrun EE, Cui T, Chatterjee S, Hofseth L, et al. Resveratrol-mediated attenuation of Staphylococcus aureus enterotoxin b-induced acute liver injury is associated with regulation of microrna and induction of myeloid-derived suppressor cells. Front Microbiol. 2018; 9:2910.
Article28. Szabo PA, Goswami A, Memarnejadian A, Mallett CL, Foster PJ, McCormick JK, et al. Swift intrahepatic accumulation of granulocytic myeloid-derived suppressor cells in a humanized mouse model of toxic shock syndrome. J Infect Dis. 2016; 213:1990–1995.
Article29. Busbee PB, Nagarkatti M, Nagarkatti PS. Natural indoles, indole-3-carbinol (I3C) and 3,3’-diindolylmethane (DIM), attenuate staphylococcal enterotoxin B-mediated liver injury by downregulating miR-31 expression and promoting caspase-2-mediated apoptosis. PLoS One. 2015; 10:e0118506.
Article30. McKallip RJ, Fisher M, Gunthert U, Szakal AK, Nagarkatti PS, Nagarkatti M. Role of CD44 and its v7 isoform in staphylococcal enterotoxin B-induced toxic shock: CD44 deficiency on hepatic mononuclear cells leads to reduced activation-induced apoptosis that results in increased liver damage. Infect Immun. 2005; 73:50–61.
Article31. Yin T, Tong SQ, Xie YC, Lu DY. Cyclosporin A protects Balb/c mice from liver damage induced by superan tigen SEB and D-GalN. World J Gastroenterol. 1999; 5:209–212.
Article32. Ogawa T, Enomoto M, Fujii H, Sekiya Y, Yoshizato K, Ikeda K, et al. MicroRNA-221/222 upregulation indicates the activation of stellate cells and the progression of liver fibrosis. Gut. 2012; 61:1600–1609.
Article33. Pineau P, Volinia S, McJunkin K, Marchio A, Battiston C, Terris B, et al. miR-221 overexpression contributes to liver tumorigenesis. Proc Natl Acad Sci U S A. 2010; 107:264–269.
Article34. Li Y, Liang C, Ma H, Zhao Q, Lu Y, Xiang Z, et al. miR-221/222 promotes S-phase entry and cellular migration in control of basal-like breast cancer. Molecules. 2014; 19:7122–7137.
Article35. Lv X, Zhang Y, Cui Y, Ren Y, Li R, Rong Q. Inhibition of microRNA-155 relieves sepsis-induced liver injury through inactivating the JAK/ STAT pathway. Mol Med Rep. 2015; 12:6013–6018.
Article36. Ren JP, Ying RS, Cheng YQ, Wang L, El Gazzar M, Li GY, et al. HCV-induced miR146a controls SOCS1/STAT3 and cytokine expression in monocytes to promote regulatory T-cell development. J Viral Hepat. 2016; 23:755–766.
Article37. Zhang H, Zhao Z, Pang X, Yang J, Yu H, Zhang Y, et al. Genistein protects against Ox-LDL-induced inflammation through microRNA-155/SOCS1-mediated repression of NF-kB signaling pathway in HUVECs. Inflammation. 2017; 40:1450–1459.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hsa-miRNA-143-3p Reverses Multidrug Resistance of Triple-Negative Breast Cancer by Inhibiting the Expression of Its Target Protein Cytokine-Induced Apoptosis Inhibitor 1 In Vivo
- Improved Differentiation Ability and Therapeutic Effect of miR-23a-3p Expressing Bone Marrow-Derived Mesenchymal Stem Cells in Mice Model with Acute Lung Injury
- Increase of Rhinovirus Replication in Airway Epithelial Cells by Staphylococcal Enterotoxin A and B
- SOCS3 Attenuates DexamethasoneInduced M2 Polarization by DownRegulation of GILZ via ROS- and p38 MAPK-Dependent Pathways
- Cytokine Inductions and Intracellular Signal Profiles by Stimulation of dsRNA and SEB in the Macrophages and Epithelial Cells