Anat Biol Anthropol.
2019 Dec;32(4):121-128. 10.11637/aba.2019.32.4.121.
Extraneural CGRP Induces Oxidative Stress in Kidney Proximal Tubule Epithelial Cells
- Affiliations
-
- 1Interdisciplinary Graduate Program in Advanced Convergence Technology & Science, Jeju National University, Korea. jinu.kim@jejunu.ac.kr
- 2Department of Anatomy, Jeju National University School of Medicine, Korea.
- 3Department of Cellular and Integrative Physiology, University of Nebraska Medical Center, Korea.
- KMID: 2467465
- DOI: http://doi.org/10.11637/aba.2019.32.4.121
Abstract
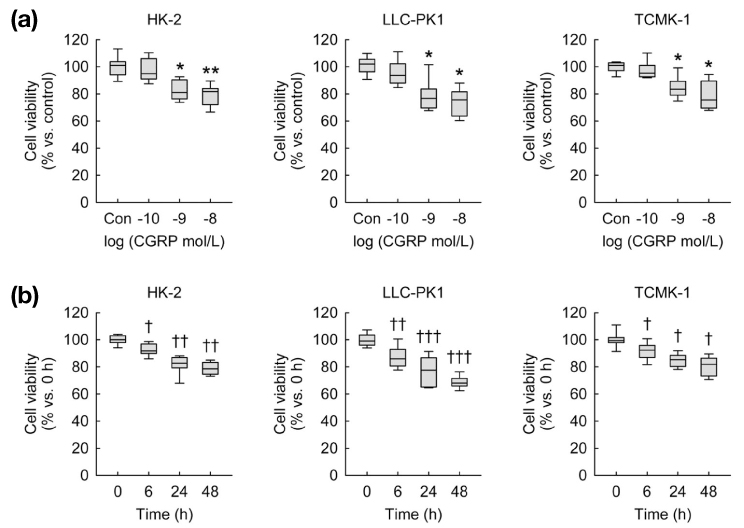
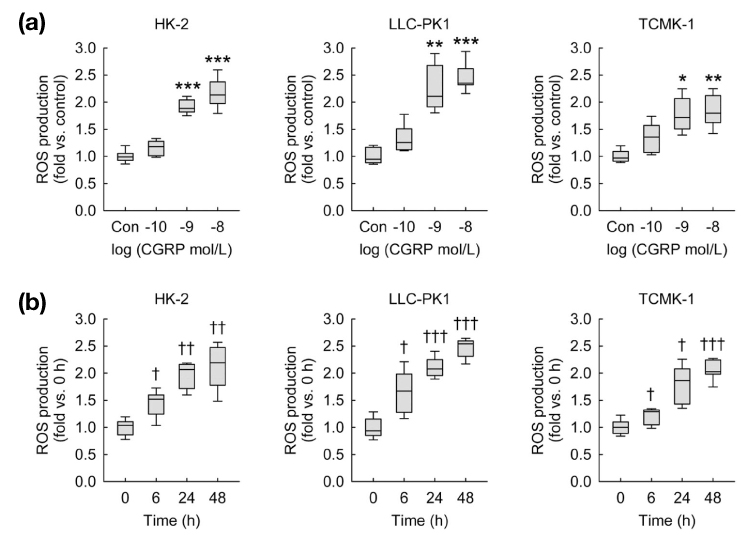
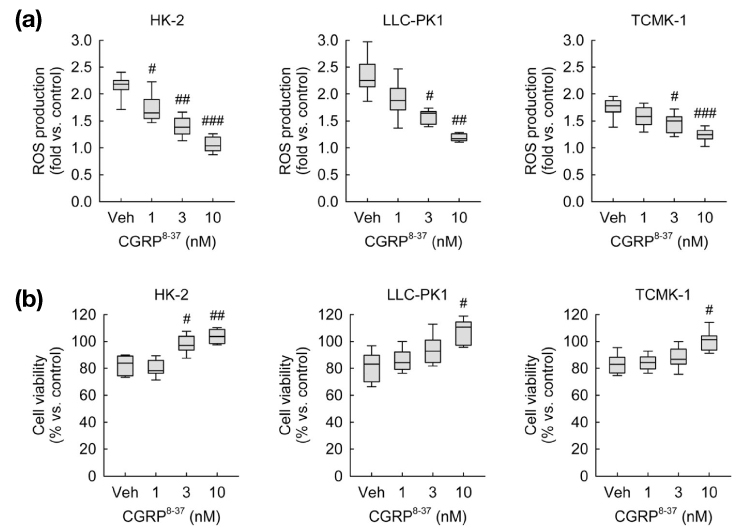
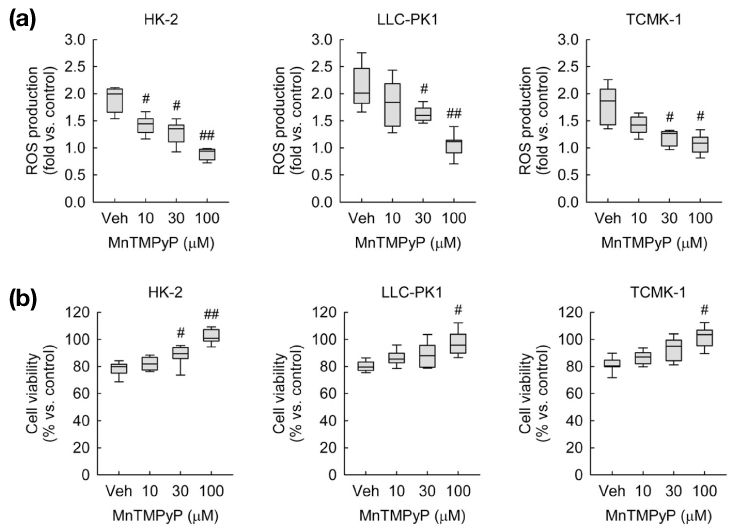
- Calcitonin gene-related peptide (CGRP) is the most abundant neuropeptide in primary afferent sensory neurons. Exogenous CGRP can induce cell death in kidney tubular cells. The objective of this study was to determine whether exogenous CGRP could induce reactive oxygen species (ROS) production in kidney proximal tubule epithelial cells and whether CGRP-induced ROS production might contribute to cell death. In HK-2, LLC-PK1 and TCMK-1 cell lines derived from human, pig, and mouse respectively, administration of CGRP increased cell death in time- and dose-dependent manners, as demonstrated by decreased cell viability. Exogenous CGRP also increased ROS production levels in those cell lines. Treatment with CGRP receptor antagonist(CGRP(8-37)) significantly inhibited the increases in cell death and ROS production in CGRP-exposed cells. Furthermore, treatment with a ROS scavenger (MnTMPyP) markedly reduced kidney proximal tubule epithelial cell death after CGRP administration. Taken together, these data suggest that extraneural CGRP can induce cell death through excessive oxidative stress in kidney proximal tubule epithelial cells.
Keyword
MeSH Terms
-
Animals
Calcitonin Gene-Related Peptide
Cell Death
Cell Line
Cell Survival
Epithelial Cells*
Humans
Kidney*
Mice
Neuropeptides
Oxidative Stress*
Reactive Oxygen Species
Receptors, Calcitonin Gene-Related Peptide
Sensory Receptor Cells
Calcitonin Gene-Related Peptide
Neuropeptides
Reactive Oxygen Species
Receptors, Calcitonin Gene-Related Peptide
Figure
Reference
-
1. Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014; 94:1099–1142.
Article2. Ferguson M, Bell C. Ultrastructural localization and characterization of sensory nerves in the rat kidney. J Comp Neurol. 1988; 274:9–16.
Article3. Watkins HA, Rathbone DL, Barwell J, Hay DL, Poyner DR. Structure-activity relationships for alpha-calcitonin gene-related peptide. Br J Pharmacol. 2013; 170:1308–1322.
Article4. Calò LA, Davis PA, Pagnin E, Dal Maso L, Caielli P, Rossi GP. Calcitonin gene-related peptide, heme oxygenase-1, endothelial progenitor cells and nitric oxide-dependent vasodilation relationships in a human model of angiotensin II type-1 receptor antagonism. J Hypertens. 2012; 30:1406–1413.
Article5. Kim J, Padanilam BJ. Renal nerves drive interstitial fibrogenesis in obstructive nephropathy. J Am Soc Nephrol. 2013; 24:229–242.
Article6. Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007; 12:913–922.
Article7. Kim J, Kim KY, Jang HS, Yoshida T, Tsuchiya K, Nitta K, et al. Role of cytosolic NADP+-dependent isocitrate dehydrogenase in ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2009; 296:F622–F633.8. Kim J, Long KE, Tang K, Padanilam BJ. Poly (ADP-ribose) polymerase 1 activation is required for cisplatin nephrotoxicity. Kidney Int. 2012; 82:193–203.
Article9. Wei Q, Dong G, Franklin J, Dong Z. The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int. 2007; 72:53–62.
Article10. Kim J, Padanilam BJ. Renal denervation prevents long-term sequelae of ischemic renal injury. Kidney Int. 2015; 87:350–358.
Article11. Kim J. Spermidine rescues proximal tubular cells from oxidative stress and necrosis after ischemic acute kidney injury. Arch Pharm Res. 2017; 40:1197–1208.
Article12. Park S, Yoon SP, Kim J. Cisplatin induces primary necrosis through poly (ADP-ribose) polymerase 1 activation in kidney proximal tubular cells. Anat Cell Biol. 2015; 48:66–74.
Article13. Lee JS, Lim JY, Kim J. Mechanical stretch induces angiotensinogen expression through PARP1 activation in kidney proximal tubular cells. In Vitro Cell Dev Biol Anim. 2015; 51:72–78.
Article14. Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999; 27:612–616.
Article15. Yoon SP, Kim J. Exogenous CGRP upregulates profibrogenic growth factors through PKC/JNK signaling pathway in kidney proximal tubular cells. Cell Biol Toxicol. 2018; 34:251–262.
Article16. Schaeffer C, Vandroux D, Thomassin L, Athias P, Rochette L, Connat JL. Calcitonin gene-related peptide partly protects cultured smooth muscle cells from apoptosis induced by an oxidative stress via activation of ERK1/2 MAPK. Biochim Biophys Acta. 2003; 1643:65–73.
Article17. Sueur S, Pesant M, Rochette L, Connat JL. Antiapoptotic effect of calcitonin gene-related peptide on oxidative stress-induced injury in H9c2 cardiomyocytes via the RAMP1/CRLR complex. J Mol Cell Cardiol. 2005; 39:955–963.
Article18. Mulderry PK, Ghatei MA, Rodrigo J, Allen JM, Rosenfeld MG, Polak JM, et al. Calcitonin gene-related peptide in cardiovascular tissues of the rat. Neuroscience. 1985; 14:947–954.
Article19. Lappe RW, Slivjak MJ, Todt JA, Wendt RL. Hemodynamic effects of calcitonin gene-related peptide in conscious rats. Regul Pept. 1987; 19:307–312.
Article20. Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, et al. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD (P)H oxidase. J Am Soc Nephrol. 2003; 14:S227–S232.21. Chambers JW, LoGrasso PV. Mitochondrial c-Jun N-terminal kinase (JNK) signaling initiates physiological changes resulting in amplification of reactive oxygen species generation. J Biol Chem. 2011; 286:16052–16062.
Article22. Neri M, Cerretani D, Fiaschi AI, Laghi PF, Lazzerini PE, Maffione AB, et al. Correlation between cardiac oxidative stress and myocardial pathology due to acute and chronic norepinephrine administration in rats. J Cell Mol Med. 2007; 11:156–170.
Article23. Bianchi P, Pimentel DR, Murphy MP, Colucci WS, Parini A. A new hypertrophic mechanism of serotonin in cardiac myocytes: receptor-independent ROS generation. FASEB J. 2005; 19:641–643.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Extraneural CGRP Upregulates TGF-β1 through RAMP1 Signaling during Mechanical Stretch in Kidney Proximal Tubule Epithelial Cells
- Regulation of Angiotensin II Binding in Renal Proximal Tubule Cells by High Glucose : I.Involvement of PKC, Ca2+, and Oxidative Stress
- Expression of Occludin in Porcine Renal Epithelial Cells
- Renal Transport of Urate
- The role of oxidative stress and hypoxia in renal disease