Korean J Physiol Pharmacol.
2019 Sep;23(5):393-402. 10.4196/kjpp.2019.23.5.393.
Antitumor profiles and cardiac electrophysiological effects of aurora kinase inhibitor ZM447439
- Affiliations
-
- 1R&D Center for Advanced Pharmaceuticals & Evaluation, Korea Institute of Toxicology, Korea Research Institute of Chemical Technology, Daejeon 34114, Korea. idkks@kitox.re.kr
- 2Fertility Center, CHA Bunding Medical Center, CHA University, Seongnam 13496, Korea.
- KMID: 2455815
- DOI: http://doi.org/10.4196/kjpp.2019.23.5.393
Abstract
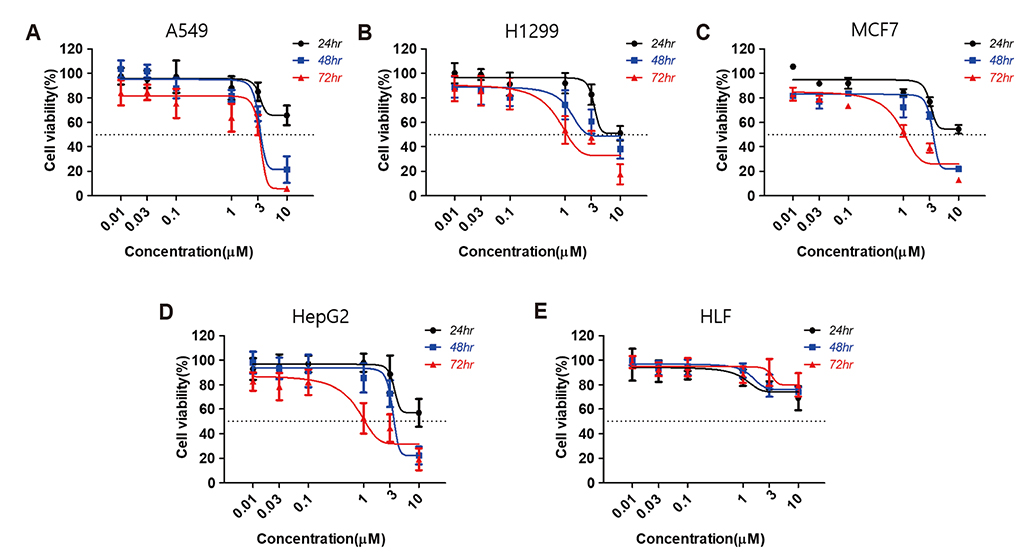
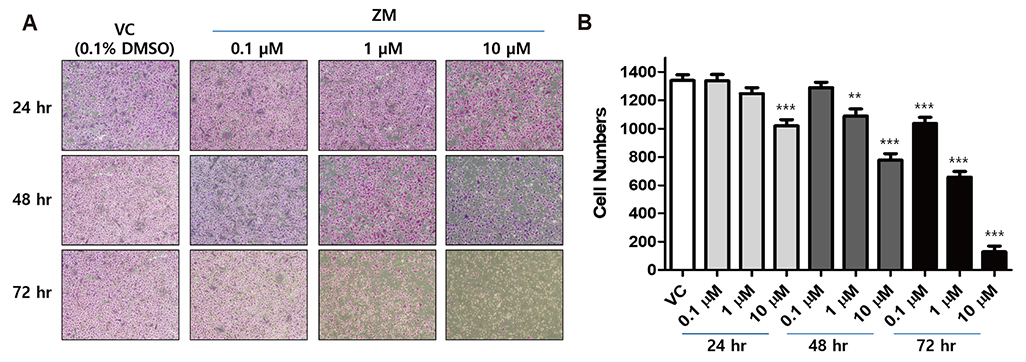
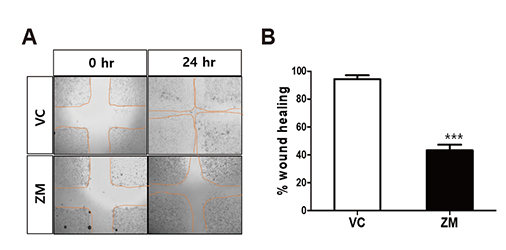
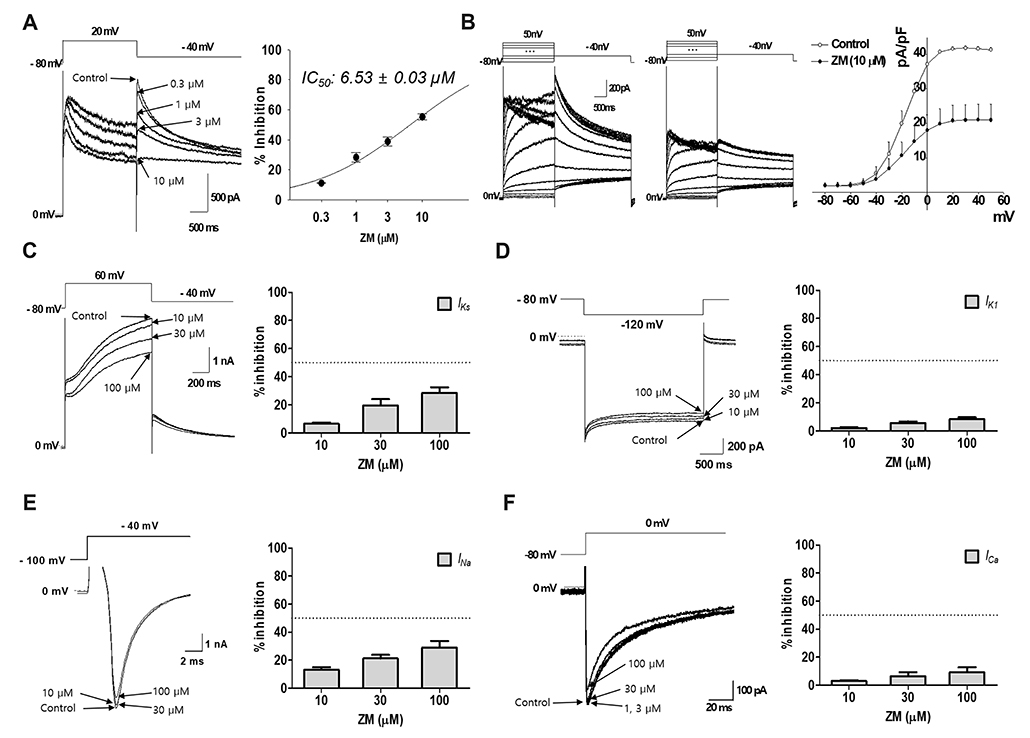
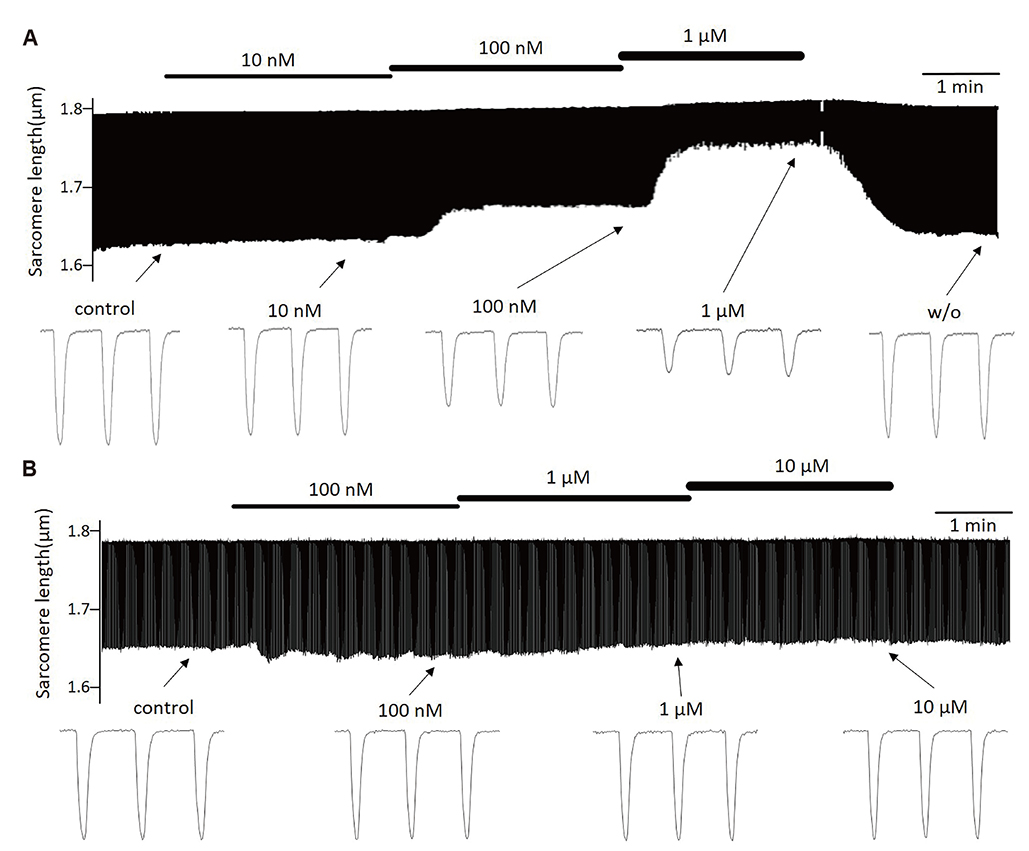
- Aurora kinases inhibitors, including ZM447439 (ZM), which suppress cell division, have attracted a great deal of attention as potential novel anti-cancer drugs. Several recent studies have confirmed the anti-cancer effects of ZM in various cancer cell lines. However, there have been no studies regarding the cardiac safety of this agent. We performed several cytotoxicity, invasion and migration assays to examine the anti-cancer effects of ZM. To evaluate the potential effects of ZM on cardiac repolarisation, whole-cell patch-clamp experiments were performed with human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) and cells with heterogeneous cardiac ion channel expression. We also conducted a contractility assay with rat ventricular myocytes to determine the effects of ZM on myocardial contraction and/or relaxation. In tests to determine in vitro efficacy, ZM inhibited the proliferation of A549, H1299 (lung cancer), MCF-7 (breast cancer) and HepG2 (hepatoma) cell lines with ICâ‚…â‚€ in the submicromolar range, and attenuated the invasive and metastatic capacity of A549 cells. In cardiac toxicity testing, ZM did not significantly affect I(Na), I(Ks) or I(K1), but decreased I(hERG) in a dose-dependent manner (ICâ‚…â‚€: 6.53 µM). In action potential (AP) assay using hiPSC-CMs, ZM did not induce any changes in AP parameters up to 3 µM, but it at 10 µM induced prolongation of AP duration. In summary, ZM showed potent broad-spectrum anti-tumor activity, but relatively low levels of cardiac side effects compared to the effective doses to tumor. Therefore, ZM has a potential to be a candidate as an anti-cancer with low cardiac toxicity.
MeSH Terms
Figure
Reference
-
1. Cirak Y, Furuncuoglu Y, Yapicier O, Aksu A, Cubukcu E. Aurora A overexpression in breast cancer patients induces taxane resistance and results in worse prognosis. J BUON. 2015; 20:1414–1419.2. Zhang Y, Jiang C, Li H, Lv F, Li X, Qian X, Fu L, Xu B, Guo X. Elevated Aurora B expression contributes to chemoresistance and poor prognosis in breast cancer. Int J Clin Exp Pathol. 2015; 8:751–757.3. Zekri A, Lesan V, Ghaffari SH, Tabrizi MH, Modarressi MH. Gene amplification and overexpression of Aurora-C in breast and prostate cancer cell lines. Oncol Res. 2012; 20:241–250.
Article4. Yang H, Ou CC, Feldman RI, Nicosia SV, Kruk PA, Cheng JQ. Aurora-A kinase regulates telomerase activity through c-Myc in human ovarian and breast epithelial cells. Cancer Res. 2004; 64:463–467.
Article5. Gavriilidis P, Giakoustidis A, Giakoustidis D. Aurora kinases and potential medical applications of aurora kinase inhibitors: a review. J Clin Med Res. 2015; 7:742–751.
Article6. Sasai K, Katayama H, Stenoien DL, Fujii S, Honda R, Kimura M, Okano Y, Tatsuka M, Suzuki F, Nigg EA, Earnshaw WC, Brinkley WR, Sen S. Aurora-C kinase is a novel chromosomal passenger protein that can complement Aurora-B kinase function in mitotic cells. Cell Motil Cytoskeleton. 2004; 59:249–263.
Article7. Yan X, Cao L, Li Q, Wu Y, Zhang H, Saiyin H, Liu X, Zhang X, Shi Q, Yu L. Aurora C is directly associated with Survivin and required for cytokinesis. Genes Cells. 2005; 10:617–626.
Article8. Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003; 161:267–280.
Article9. Woo JK, Kang JH, Shin D, Park SH, Kang K, Nho CW, Seong JK, Lee SJ, Oh SH. Daurinol enhances the efficacy of radiotherapy in lung cancer via suppression of aurora kinase A/B expression. Mol Cancer Ther. 2015; 14:1693–1704.
Article10. Ding YH, Zhou ZW, Ha CF, Zhang XY, Pan ST, He ZX, Edelman JL, Wang D, Yang YX, Zhang X, Duan W, Yang T, Qiu JX, Zhou SF. Alisertib, an Aurora kinase A inhibitor, induces apoptosis and autophagy but inhibits epithelial to mesenchymal transition in human epithelial ovarian cancer cells. Drug Des Devel Ther. 2015; 9:425–464.11. Min YH, Kim W, Kim JE. The Aurora kinase A inhibitor TC-A2317 disrupts mitotic progression and inhibits cancer cell proliferation. Oncotarget. 2016; 7:84718–84735.
Article12. Li M, Jung A, Ganswindt U, Marini P, Friedl A, Daniel PT, Lauber K, Jendrossek V, Belka C. Aurora kinase inhibitor ZM447439 induces apoptosis via mitochondrial pathways. Biochem Pharmacol. 2010; 79:122–129.
Article13. Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007; 7:332–344.
Article14. Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, Ruhsam M, Hejna M, Schmidinger H. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008; 26:5204–5212.
Article15. Sereno M, Brunello A, Chiappori A, Barriuso J, Casado E, Belda C, de Castro J, Feliu J, González-Barón M. Cardiac toxicity: old and new issues in anti-cancer drugs. Clin Transl Oncol. 2008; 10:35–46.
Article16. Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol. 2015; 12:547–558.
Article17. Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, Cipolla CM. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J Clin. 2016; 66:309–325.
Article18. Peroukides S, Alexopoulos A, Kalofonos H, Papadaki H. Cardiovascular effects of treatment with taxanes. J Cardiovasc Med (Hagerstown). 2012; 13:319–324.
Article19. Willems E, Dedobbeleer M, Digregorio M, Lombard A, Lumapat PN, Rogister B. The functional diversity of Aurora kinases: a comprehensive review. Cell Div. 2018; 13:7.
Article20. Walter AO, Seghezzi W, Korver W, Sheung J, Lees E. The mitotic serine/threonine kinase Aurora2/AIK is regulated by phosphorylation and degradation. Oncogene. 2000; 19:4906–4916.
Article21. Margolis RL, Lohez OD, Andreassen PR. G1 tetraploidy checkpoint and the suppression of tumorigenesis. J Cell Biochem. 2003; 88:673–683.
Article22. Sinnecker D, Laugwitz KL, Moretti A. Induced pluripotent stem cell-derived cardiomyocytes for drug development and toxicity testing. Pharmacol Ther. 2014; 143:246–252.
Article23. Lynch S, Pridgeon CS, Duckworth CA, Sharma P, Park BK, Goldring CEP. Stem cell models as an in vitro model for predictive toxicology. Biochem J. 2019; 476:1149–1158.24. Morgan JP, Morgan KG. Calcium and cardiovascular function. Intracellular calcium levels during contraction and relaxation of mammalian cardiac and vascular smooth muscle as detected with aequorin. Am J Med. 1984; 77(5A):33–46.25. Bodi I, Mikala G, Koch SE, Akhter SA, Schwartz A. The L-type calcium channel in the heart: the beat goes on. J Clin Invest. 2005; 115:3306–3317.
Article26. Mladěnka P, Applová L, Patoáka J, Costa VM, Remiao F, Pourová J, Mladěnka A, Karlíčková J, Jahodář L, Vopršalová M, Varner KJ, Štěrba M. Comprehensive review of cardiovascular toxicity of drugs and related agents. Med Res Rev. 2018; 38:1332–1403.
Article27. Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006; 440:463–469.
Article28. Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004; 350:1013–1022.
Article29. Li Z, Dutta S, Sheng J, Tran PN, Wu W, Chang K, Mdluli T, Strauss DG, Colatsky T. Improving the in silico assessment of proarrhythmia risk by combining hERG (Human Ether-à-go-go-Related Gene) channel-drug binding kinetics and multichannel pharmacology. Circ Arrhythm Electrophysiol. 2017; 10:e004628.
Article30. Brugada R, Hong K, Dumaine R, Cordeiro J, Gaita F, Borggrefe M, Menendez TM, Brugada J, Pollevick GD, Wolpert C, Burashnikov E, Matsuo K, Wu YS, Guerchicoff A, Bianchi F, Giustetto C, Schimpf R, Brugada P, Antzelevitch C. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation. 2004; 109:30–35.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characterization of the Indirubin Derivative LDD970 as a Small Molecule Aurora Kinase A Inhibitor in Human Colorectal Cancer Cells
- Elevated Aurora Kinase A Protein Expression in Diabetic Skin Tissue
- IMMUNOHISTOCHEMICAL STUDY OF AURORA-2 KINASE IN THE ORAL SQUAMOUS CELL CARCINOMA
- Suppression of Aurora-A oncogenic potential by c-Myc downregulation
- Aurora A kinase expression is increased in leukemia stem cells, and a selective Aurora A kinase inhibitor enhances Ara-C-induced apoptosis in acute myeloid leukemia stem cells