Cancer Res Treat.
2019 Jul;51(3):886-900. 10.4143/crt.2018.375.
Jab1 Silencing Inhibits Proliferation and Sensitizes to Cisplatin in Biliary Tract Cancer
- Affiliations
-
- 1Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. ohdoyoun@snu.ac.kr
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2454281
- DOI: http://doi.org/10.4143/crt.2018.375
Abstract
- PURPOSE
Jab1 is a coactivator of c-Jun that enhances the transcriptional function of c-Jun. Jab1 is frequently overexpressed in various cancers and is associatedwith poor prognosis of cancer patients. Thus, Jab1 could be a potential therapeutic target in cancer. However, the role of Jab1 in biliary tract cancer (BTC) has not been studied.
MATERIALS AND METHODS
We performed in vitro and in vivo experiments to evaluate the therapeutic potential ofJab1 inhibition in BTC.
RESULTS
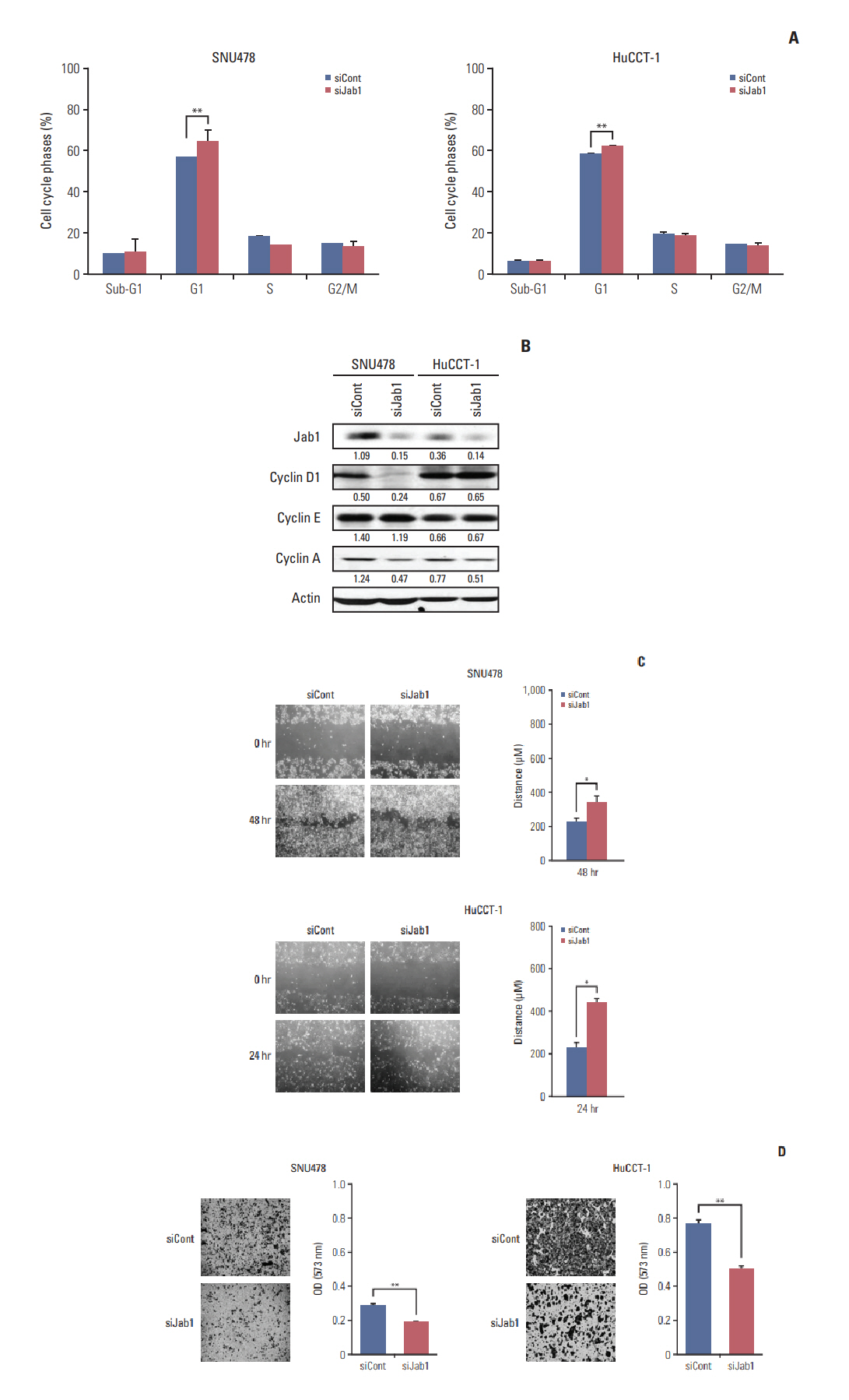
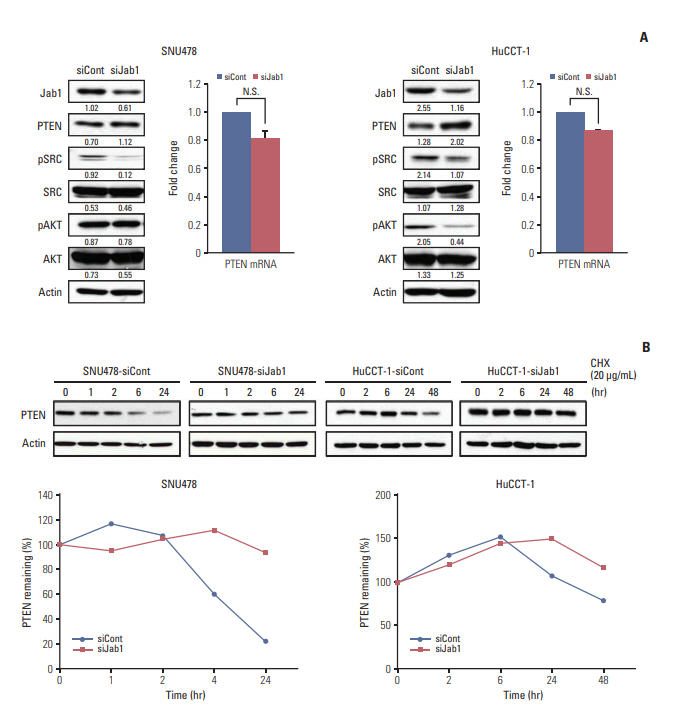
Among 8 BTC cell lines, many showed higher Jab1 expression levels. In addition, Jab1 silencing by siRNA increased p27 expression levels. SNU478 and HuCCT-1 cells exhibited profound Jab1 knockdown and increased p27 expression by Jab1-specific siRNA transfection. Jab1 silencing induced anti-proliferative and anti-migratory effects and resulted in G1 cell cycle arrest in SNU478 and HuCCT-1 cells. In addition, Jab1 silencing potentiated the anti-proliferative and anti-migratory effects of cisplatin by increasing DNA damage. Interestingly,Jab1 knockdown increased PTEN protein half-life, resulting in increased PTEN expression. In the HuCCT-1 mouse xenograft model, stable knockdown of Jab1 by shRNA also showed anti-proliferative effects in vivo, with decreased Ki-67 expression and AKT phosphorylation and increased Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling and p27 expression.
CONCLUSION
Jab1 knockdown demonstrated anti-proliferative and anti-migratory effects in BTC cells by increasing DNA damage and stabilizing PTEN, resulting in G1 cell cycle arrest. In addition, Jab1 silencing potentiated the anti-proliferative effects of cisplatin. Our data suggest that Jab1 may be a potential therapeutic target in BTC that is worthy of further investigations.
MeSH Terms
Figure
Reference
-
References
1. Claret FX, Hibi M, Dhut S, Toda T, Karin M. A new group of conserved coactivators that increase the specificity of AP-1 transcription factors. Nature. 1996; 383:453–7.
Article2. Tomoda K, Kubota Y, Kato J. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature. 1999; 398:160–5.
Article3. Tomoda K, Kubota Y, Arata Y, Mori S, Maeda M, Tanaka T, et al. The cytoplasmic shuttling and subsequent degradation of p27Kip1 mediated by Jab1/CSN5 and the COP9 signalosome complex. J Biol Chem. 2002; 277:2302–10.
Article4. Oh W, Lee EW, Sung YH, Yang MR, Ghim J, Lee HW, et al. Jab1 induces the cytoplasmic localization and degradation of p53 in coordination with Hdm2. J Biol Chem. 2006; 281:17457–65.
Article5. Lee EW, Oh W, Song J. Jab1 as a mediator of nuclear export and cytoplasmic degradation of p53. Mol Cells. 2006; 22:133–40.6. Esteva FJ, Sahin AA, Rassidakis GZ, Yuan LX, Smith TL, Yang Y, et al. Jun activation domain binding protein 1 expression is associated with low p27(Kip1)levels in node-negative breast cancer. Clin Cancer Res. 2003; 9:5652–9.7. Wang Y, Wang Y, Cheng C, Ji Y, Zhao Y, Zou L, et al. Expression of Jun activation domain-binding protein 1 and Ser10 phosphorylated p27 protein in human epithelial ovarian carcinoma. J Cancer Res Clin Oncol. 2009; 135:951–9.
Article8. Wang Y, Yu YN, Song S, Li TJ, Xiang JY, Zhang H, et al. JAB1 and phospho-Ser10 p27 expression profile determine human hepatocellular carcinoma prognosis. J Cancer Res Clin Oncol. 2014; 140:969–78.
Article9. Wang F, Wang Y, Yu X, Yang D, Wang Z, Lu C, et al. Significance of Jab1 expression in human esophageal squamous cell carcinoma. J Clin Gastroenterol. 2009; 43:520–6.
Article10. Dong Y, Sui L, Watanabe Y, Yamaguchi F, Hatano N, Tokuda M. Prognostic significance of Jab1 expression in laryngeal squamous cell carcinomas. Clin Cancer Res. 2005; 11:259–66.11. Shackleford TJ, Claret FX. JAB1/CSN5: a new player in cell cycle control and cancer. Cell Div. 2010; 5:26.
Article12. Randi G, Malvezzi M, Levi F, Ferlay J, Negri E, Franceschi S, et al. Epidemiology of biliary tract cancers: an update. Ann Oncol. 2009; 20:146–59.
Article13. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010; 362:1273–81.
Article14. Ku JL, Yoon KA, Kim IJ, Kim WH, Jang JY, Suh KS, et al. Establishment and characterisation of six human biliary tract cancer cell lines. Br J Cancer. 2002; 87:187–93.
Article15. Nam AR, Kim JW, Park JE, Bang JH, Jin MH, Lee KH, et al. Src as a therapeutic target in biliary tract cancer. Mol Cancer Ther. 2016; 15:1515–24.
Article16. Jin MH, Nam AR, Park JE, Bang JH, Bang YJ, Oh DY. Resistance mechanism against trastuzumab in HER2-positive cancer cells and its negation by Src inhibition. Mol Cancer Ther. 2017; 16:1145–54.
Article17. Zhang S, Huang WC, Li P, Guo H, Poh SB, Brady SW, et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med. 2011; 17:461–9.
Article18. Tian L, Peng G, Parant JM, Leventaki V, Drakos E, Zhang Q, et al. Essential roles of Jab1 in cell survival, spontaneous DNA damage and DNA repair. Oncogene. 2010; 29:6125–37.
Article19. Fukumoto A, Tomoda K, Yoneda-Kato N, Nakajima Y, Kato JY. Depletion of Jab1 inhibits proliferation of pancreatic cancer cell lines. FEBS Lett. 2006; 580:5836–44.
Article20. Schutz AK, Hennes T, Jumpertz S, Fuchs S, Bernhagen J. Role of CSN5/JAB1 in Wnt/beta-catenin activation in colorectal cancer cells. FEBS Lett. 2012; 586:1645–51.21. Sang MM, Du WQ, Zhang RY, Zheng JN, Pei DS. Suppression of CSN5 promotes the apoptosis of gastric cancer cells through regulating p53-related apoptotic pathways. Bioorg Med Chem Lett. 2015; 25:2897–901.
Article22. Pan Y, Zhang Q, Tian L, Wang X, Fan X, Zhang H, et al. Jab1/CSN5 negatively regulates p27 and plays a role in the pathogenesis of nasopharyngeal carcinoma. Cancer Res. 2012; 72:1890–900.
Article23. Rivard N, L'Allemain G, Bartek J, Pouyssegur J. Abrogation of p27Kip1 by cDNA antisense suppresses quiescence (G0 state) in fibroblasts. J Biol Chem. 1996; 271:18337–41.24. Pan Y, Zhang Q, Atsaves V, Yang H, Claret FX. Suppression of Jab1/CSN5 induces radio- and chemo-sensitivity in nasopharyngeal carcinoma through changes to the DNA damage and repair pathways. Oncogene. 2013; 32:2756–66.
Article25. Zhong G, Li H, Shan T, Zhang N. CSN5 silencing inhibits invasion and arrests cell cycle progression in human colorectal cancer SW480 and LS174T cells in vitro. Int J Clin Exp Pathol. 2015; 8:2809–15.26. Hsu MC, Chang HC, Hung WC. HER-2/neu transcriptionally activates Jab1 expression via the AKT/beta-catenin pathway in breast cancer cells. Endocr Relat Cancer. 2007; 14:655–67.27. Hsu MC, Chai CY, Hou MF, Chang HC, Chen WT, Hung WC. Jab1 is overexpressed in human breast cancer and is a downstream target for HER-2/neu. Mod Pathol. 2008; 21:609–16.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- SP1-induced lncRNA MCF2L-AS1 promotes cisplatin resistance in ovarian cancer by regulating IGF2BP1/IGF2/MEK/ERK axis
- The Relevance of Women's Diseases, Jun Activation-domain Binding Protein 1 (JAB1) and p27(kip1)
- Chemotherapy for Biliary Tract Cancer
- Current status of chemotherapy for the treatment of advanced biliary tract cancer
- Epigenetic modification of α-N-acetylgalactosaminidase enhances cisplatin resistance in ovarian cancer