Korean J Physiol Pharmacol.
2018 Jan;22(1):43-51. 10.4196/kjpp.2018.22.1.43.
Epigenetic modification of α-N-acetylgalactosaminidase enhances cisplatin resistance in ovarian cancer
- Affiliations
-
- 1Department of Biochemistry, School of Medicine, Ewha Womans University, Seoul 07985, Korea. ahnj@ewha.ac.kr
- 2Department of Biomedical Sciences, Seoul National University, College of Medicine, Seoul 03080, Korea.
- 3Department of Obstetrics and Gynecology, School of Medicine, Ewha Womans University Seoul 07985, Korea. goodmorning@ewha.ac.kr
- KMID: 2398554
- DOI: http://doi.org/10.4196/kjpp.2018.22.1.43
Abstract
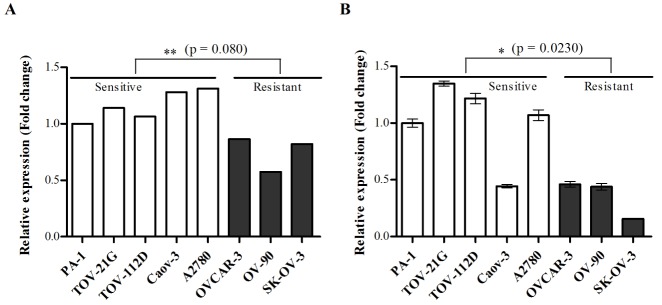
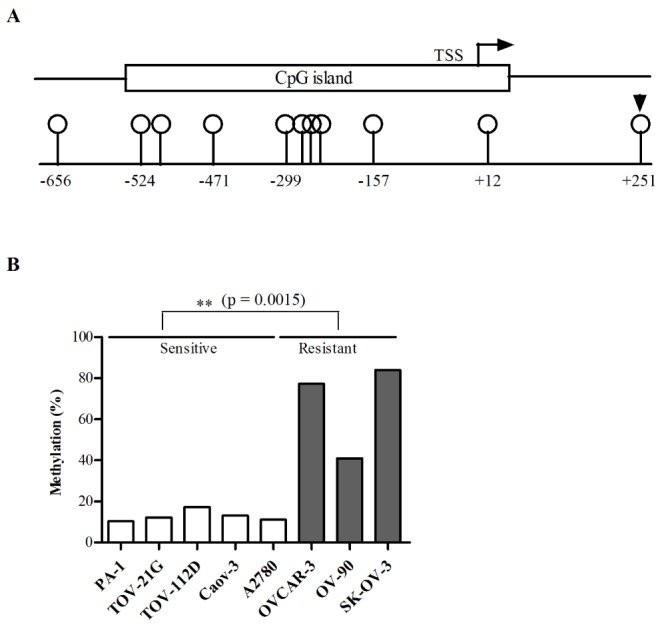
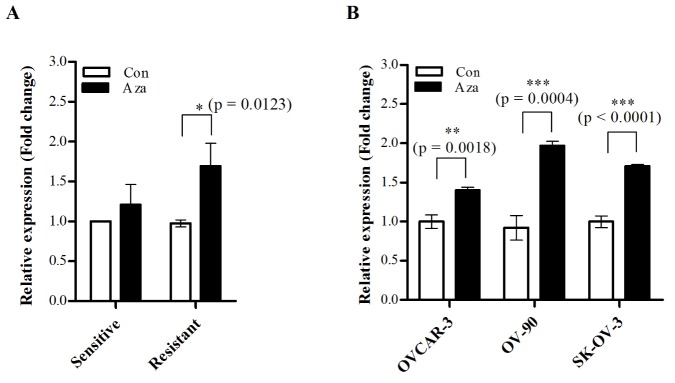
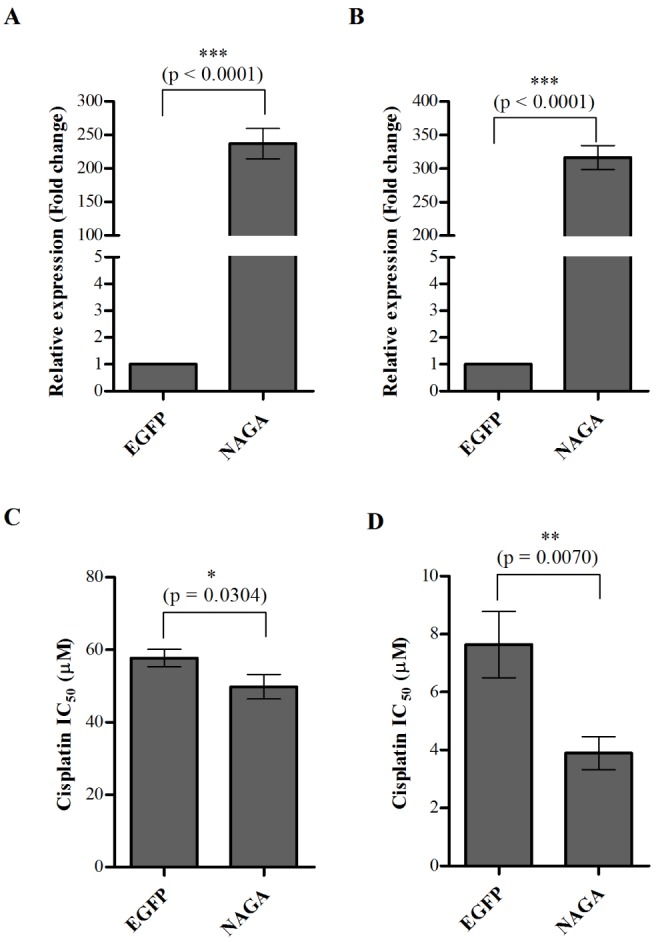
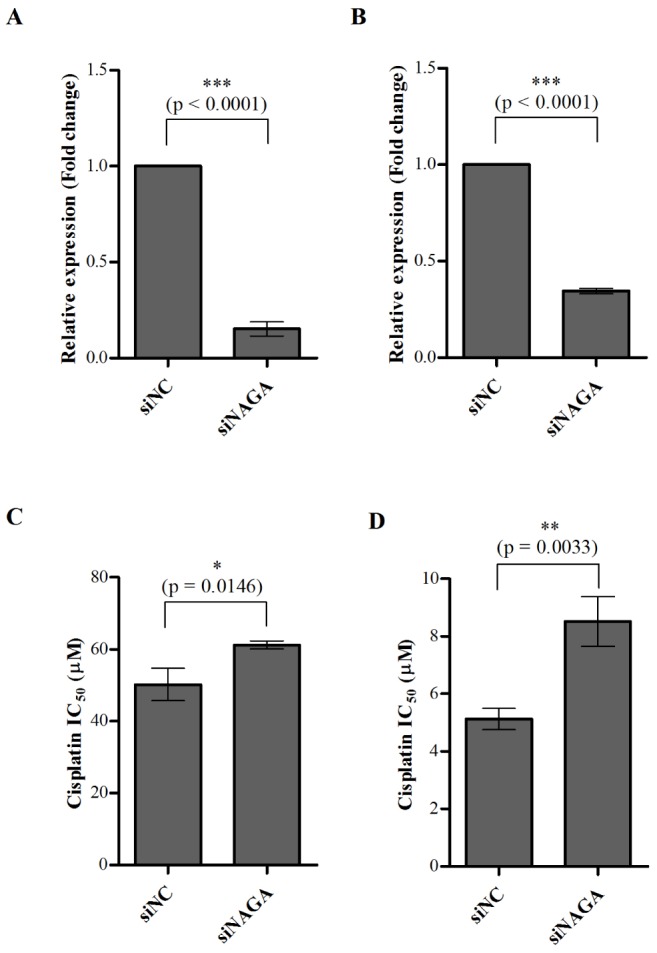
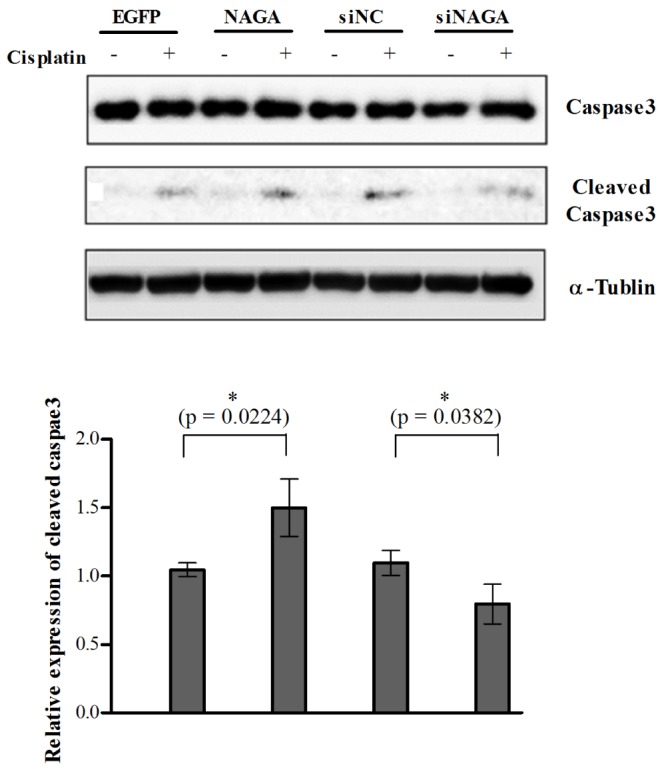
- Although cisplatin is one of the most effective antitumor drugs for ovarian cancer, the emergence of chemoresistance to cisplatin in over 80% of initially responsive patients is a major barrier to successful therapy. The precise mechanisms underlying the development of cisplatin resistance are not fully understood, but alteration of DNA methylation associated with aberrant gene silencing may play a role. To identify epigenetically regulated genes directly associated with ovarian cancer cisplatin resistance, we compared the expression and methylation profiles of cisplatin-sensitive and -resistant human ovarian cancer cell lines. We identified α-Nacetylgalactosaminidase (NAGA) as one of the key candidate genes for cisplatin drug response. Interestingly, in cisplatin-resistant cell lines, NAGA was significantly downregulated and hypermethylated at a promoter CpG site at position +251 relative to the transcriptional start site. Low NAGA expression in cisplatin-resistant cell lines was restored by treatment with a DNA demethylation agent, indicating transcriptional silencing by hyper-DNA methylation. Furthermore, overexpression of NAGA in cisplatin-resistant lines induced cytotoxicity in response to cisplatin, whereas depletion of NAGA expression increased cisplatin chemoresistance, suggesting an essential role of NAGA in sensitizing ovarian cells to cisplatin. These findings indicate that NAGA acts as a cisplatin sensitizer and its gene silencing by hypermethylation confers resistance to cisplatin in ovarian cancer. Therefore, we suggest NAGA may be a promising potential therapeutic target for improvement of sensitivity to cisplatin in ovarian cancer.
MeSH Terms
Figure
Reference
-
1. Kipps E, Tan DS, Kaye SB. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev Cancer. 2013; 13:273–282. PMID: 23426401.
Article2. Salani R, Backes FJ, Fung MF, Holschneider CH, Parker LP, Bristow RE, Goff BA. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am J Obstet Gynecol. 2011; 204:466–478. PMID: 21752752.
Article3. Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003; 3:502–516. PMID: 12835670.
Article4. Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003; 22:7265–7279. PMID: 14576837.
Article5. Fuertes MA, Castilla J, Alonso C, Pérez JM. Novel concepts in the development of platinum antitumor drugs. Curr Med Chem Anticancer Agents. 2002; 2:539–551. PMID: 12678734.
Article6. Gonzalez VM, Fuertes MA, Alonso C, Perez JM. Is cisplatin-induced cell death always produced by apoptosis? Mol Pharmacol. 2001; 59:657–663. PMID: 11259608.
Article7. Niedner H, Christen R, Lin X, Kondo A, Howell SB. Identification of genes that mediate sensitivity to cisplatin. Mol Pharmacol. 2001; 60:1153–1160. PMID: 11723219.
Article8. Chang X, Monitto CL, Demokan S, Kim MS, Chang SS, Zhong X, Califano JA, Sidransky D. Identification of hypermethylated genes associated with cisplatin resistance in human cancers. Cancer Res. 2010; 70:2870–2879. PMID: 20215521.
Article9. Wermann H, Stoop H, Gillis AJ, Honecker F, van Gurp RJ, Ammerpohl O, Richter J, Oosterhuis JW, Bokemeyer C, Looijenga LH. Global DNA methylation in fetal human germ cells and germ cell tumours: association with differentiation and cisplatin resistance. J Pathol. 2010; 221:433–442. PMID: 20593487.
Article10. Yamamoto N, Naraparaju VR, Moore M, Brent LH. Deglycosylation of serum vitamin D3-binding protein by α-N-acetylgalactosaminidase detected in the plasma of patients with systemic lupus erythematosus. Clin Immunol Immnopathol. 1997; 82:290–298.
Article11. Reddi AL, Sankaranarayanan K, Arulraj HS, Devaraj N, Devaraj H. Serum α-N-acetylgalactosaminidase is associated with diagnosis/prognosis of patients with squamous cell carcinoma of the uterine cervix. Cancer Lett. 2000; 158:61–64. PMID: 10940510.
Article12. Yamamoto N, Suyama H, Yamamoto N. Immunotherapy for Prostate Cancer with Gc Protein-Derived Macrophage-Activating Factor, GcMAF. Transl Oncol. 2008; 1:65–72. PMID: 18633461.
Article13. Thyer L, Ward E, Smith R, Branca JJ, Morucci G, Gulisano M, Noakes D, Eslinger R, Pacini S. GC protein-derived macrophage-activating factor decreases α-N-acetylgalactosaminidase levels in advanced cancer patients. Oncoimmunology. 2013; 2:e25769. PMID: 24179708.
Article14. Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004; 3:3.
Article15. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995; 57:289–300.
Article16. Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev. 2012; 64:706–721. PMID: 22659329.
Article17. Zeller C, Dai W, Steele NL, Siddiq A, Walley AJ, Wilhelm-Benartzi CS, Rizzo S, van der Zee A, Plumb JA, Brown R. Candidate DNA methylation drivers of acquired cisplatin resistance in ovarian cancer identified by methylome and expression profiling. Oncogene. 2012; 31:4567–4576. PMID: 22249249.
Article18. Jacob F, Hitchins MP, Fedier A, Brennan K, Nixdorf S, Hacker NF, Ward R, Heinzelmann-Schwarz VA. Expression of GBGT1 is epigenetically regulated by DNA methylation in ovarian cancer cells. BMC Mol Biol. 2014; 15:24. PMID: 25294702.
Article19. Basu A, Krishnamurthy S. Cellular responses to cisplatin-induced DNA damage. J Nucleic Acids. 2010; 2010:201367. PMID: 20811617.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of p53, p16, PTEN, and c-myc Gene with Cisplatin Treatment in Cisplatin Resistant Ovarian Cancer Cell Line
- SP1-induced lncRNA MCF2L-AS1 promotes cisplatin resistance in ovarian cancer by regulating IGF2BP1/IGF2/MEK/ERK axis
- Differences of EDR Chemoresistance Assay and Prognosis between Recurrent Micropapillary Serous Ovarian Carcinoma and Serous Ovarian Carcinoma
- MiR-338-3p Enhances Ovarian Cancer Cell Sensitivity to Cisplatin by Downregulating WNT2B
- Intraperitoneal cisplatin chemotherapy for advanced ovarian cancer