Tuberc Respir Dis.
2019 Apr;82(2):133-142. 10.4046/trd.2017.0124.
Bleomycin Inhibits Proliferation via Schlafen-Mediated Cell Cycle Arrest in Mouse Alveolar Epithelial Cells
- Affiliations
-
- 1Department of Thoracic and Cardiovascular Surgery, Kangwon National University School of Medicine, Chuncheon, Korea. seran@kangwon.ac.kr
- 2Department of Internal Medicine, Kangwon National University School of Medicine, Chuncheon, Korea.
- 3Department of Molecular and Cellular Biochemistry, Kangwon National University School of Medicine, Chuncheon, Korea.
- 4Department of Physiology, Kangwon National University School of Medicine, Chuncheon, Korea.
- 5Department of Medical Environmental Biology and Tropical Medicine, Kangwon National University School of Medicine, Chuncheon, Korea.
- KMID: 2453983
- DOI: http://doi.org/10.4046/trd.2017.0124
Abstract
- BACKGROUND
Idiopathic pulmonary fibrosis involves irreversible alveolar destruction. Although alveolar epithelial type II cells are key functional participants within the lung parenchyma, how epithelial cells are affected upon bleomycin (BLM) exposure remains unknown. In this study, we determined whether BLM could induce cell cycle arrest via regulation of Schlafen (SLFN) family genes, a group of cell cycle regulators known to mediate growth-inhibitory responses and apoptosis in alveolar epithelial type II cells.
METHODS
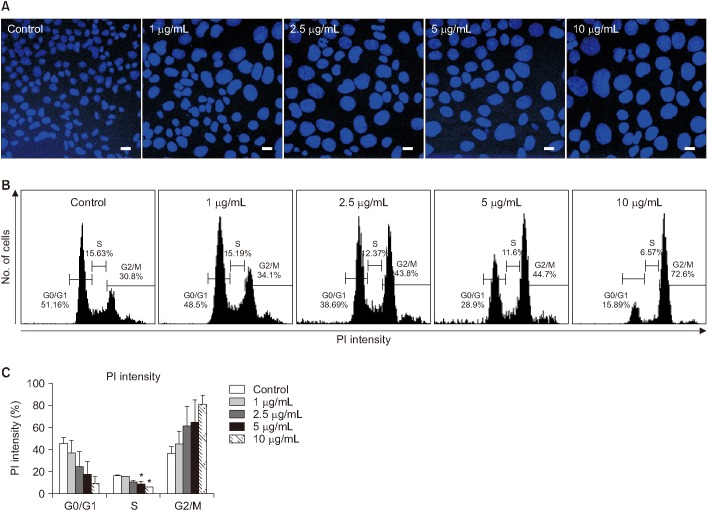
Mouse AE II cell line MLE-12 were exposed to 1-10 µg/mL BLM and 0.01-100 µM baicalein (Bai), a G1/G2 cell cycle inhibitor, for 24 hours. Cell viability and levels of pro-inflammatory cytokines were analyzed by MTT and enzyme-linked immunosorbent assay, respectively. Apoptosis-related gene expression was evaluated by quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR). Cellular morphology was determined after DAPI and Hoechst 33258 staining. To verify cell cycle arrest, propidium iodide (PI) staining was performed for MLE-12 after exposure to BLM.
RESULTS
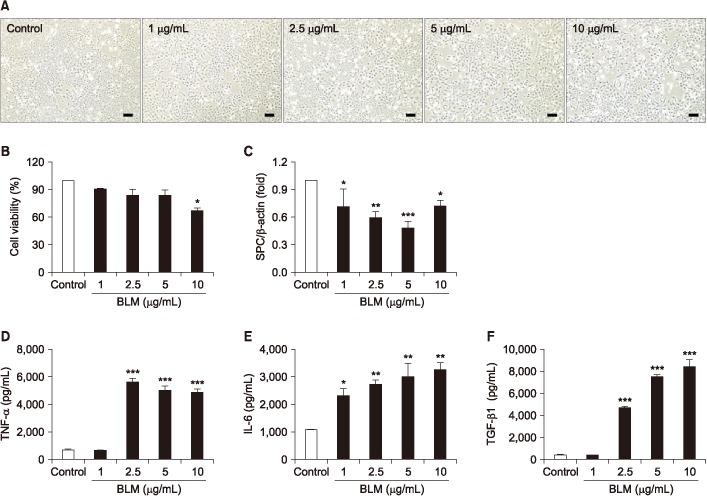
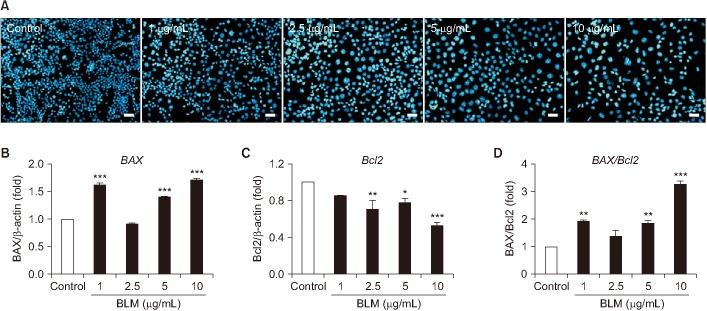
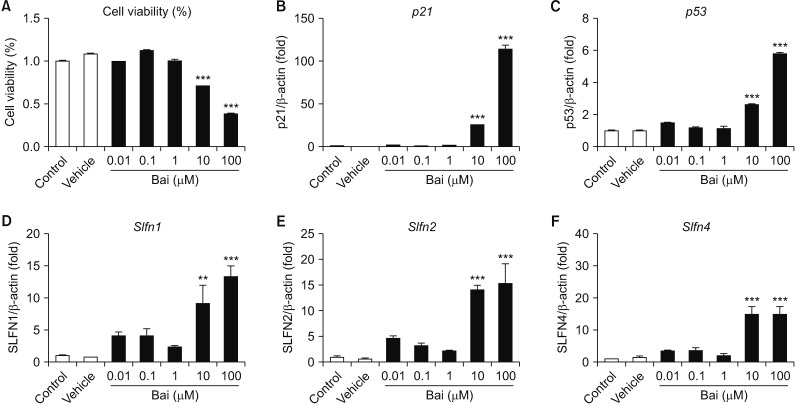
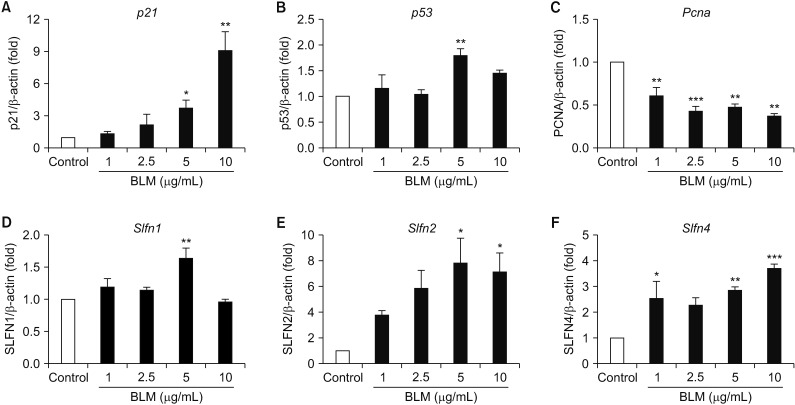
BLM decreased the proliferation of MLE-12 cells. However, it significantly increased expression levels of interleukin 6, tumor necrosis factor α, and transforming growth factor β1. Based on Hoechst 33258 staining, BLM induced condensation of nuclear and fragmentation. Based on DAPI and PI staining, BLM significantly increased the size of nuclei and induced G2/M phase cell cycle arrest. Results of qRT-PCR analysis revealed that BLM increased mRNA levels of BAX but decreased those of Bcl2. In addition, BLM/Bai increased mRNA levels of p53, p21, SLFN1, 2, 4 of Schlafen family.
CONCLUSION
BLM exposure affects pulmonary epithelial type II cells, resulting in decreased proliferation possibly through apoptotic and cell cycle arrest associated signaling.
Keyword
MeSH Terms
-
Animals
Apoptosis
Bisbenzimidazole
Bleomycin*
Cell Cycle Checkpoints*
Cell Cycle*
Cell Line
Cell Survival
Cytokines
Enzyme-Linked Immunosorbent Assay
Epithelial Cells*
Gene Expression
Genes, vif
Humans
Idiopathic Pulmonary Fibrosis
Interleukin-6
Lung
Mice*
Propidium
RNA, Messenger
Transforming Growth Factors
Tumor Necrosis Factor-alpha
Bisbenzimidazole
Bleomycin
Cytokines
Interleukin-6
Propidium
RNA, Messenger
Transforming Growth Factors
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011; 378:1949–1961. PMID: 21719092.
Article2. Yao R, He Y, Zeng Z, Liang Z, Cao Y. Protective effect of adiponectin on paraquat-induced pulmonary fibrosis in mice. Mol Cell Toxicol. 2015; 11:247–255.
Article3. Borchers AT, Chang C, Keen CL, Gershwin ME. Idiopathic pulmonary fibrosis: an epidemiological and pathological review. Clin Rev Allergy Immunol. 2011; 40:117–134. PMID: 20838937.4. Hardie WD, Hagood JS, Dave V, Perl AK, Whitsett JA, Korfhagen TR, et al. Signaling pathways in the epithelial origins of pulmonary fibrosis. Cell Cycle. 2010; 9:2769–2776. PMID: 20676040.
Article5. Romero Y, Bueno M, Ramirez R, Alvarez D, Sembrat JC, Goncharova EA, et al. mTORC1 activation decreases autophagy in aging and idiopathic pulmonary fibrosis and contributes to apoptosis resistance in IPF fibroblasts. Aging Cell. 2016; 15:1103–1112. PMID: 27566137.6. Tanaka Y, Ishitsuka Y, Hayasaka M, Yamada Y, Miyata K, Endo M, et al. The exacerbating roles of CCAAT/enhancer-binding protein homologous protein (CHOP) in the development of bleomycin-induced pulmonary fibrosis and the preventive effects of tauroursodeoxycholic acid (TUDCA) against pulmonary fibrosis in mice. Pharmacol Res. 2015; 99:52–62. PMID: 26005208.7. Jablonski RP, Kim SJ, Cheresh P, Williams DB, Morales-Nebreda L, Cheng Y, et al. SIRT3 deficiency promotes lung fibrosis by augmenting alveolar epithelial cell mitochondrial DNA damage and apoptosis. FASEB J. 2017; 31:2520–2532. PMID: 28258190.
Article8. Puglisi S, Torrisi SE, Vindigni V, Giuliano R, Palmucci S, Mule M, et al. New perspectives on management of idiopathic pulmonary fibrosis. Ther Adv Chronic Dis. 2016; 7:108–120. PMID: 26977280.
Article9. Hay J, Shahzeidi S, Laurent G. Mechanisms of bleomycininduced lung damage. Arch Toxicol. 1991; 65:81–94. PMID: 1711838.
Article10. Tang H, Gao L, Mao J, He H, Liu J, Cai X, et al. Salidroside protects against bleomycin-induced pulmonary fibrosis: activation of Nrf2-antioxidant signaling, and inhibition of NF-κB and TGF-β1/Smad-2/-3 pathways. Cell Stress Chaperones. 2016; 21:239–249. PMID: 26577463.
Article11. Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc. 2006; 3:364–372. PMID: 16738202.
Article12. Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010; 181:254–263. PMID: 19850947.
Article13. Huang TT, Lai HC, Ko YF, Ojcius DM, Lan YW, Martel J, et al. Hirsutella sinensis mycelium attenuates bleomycin-induced pulmonary inflammation and fibrosis in vivo. Sci Rep. 2015; 5:15282. PMID: 26497260.
Article14. Wang Y, Li R, Chen L, Tan W, Sun Z, Xia H, et al. Maresin 1 inhibits epithelial-to-mesenchymal transition in vitro and attenuates bleomycin induced lung fibrosis in vivo. Shock. 2015; 44:496–502. PMID: 26196843.15. Guan R, Wang X, Zhao X, Song N, Zhu J, Wang J, et al. Emodin ameliorates bleomycin-induced pulmonary fibrosis in rats by suppressing epithelial-mesenchymal transition and fibroblast activation. Sci Rep. 2016; 6:35696. PMID: 27774992.
Article16. Okudela K, Ito T, Mitsui H, Hayashi H, Udaka N, Kanisawa M, et al. The role of p53 in bleomycin-induced DNA damage in the lung: a comparative study with the small intestine. Am J Pathol. 1999; 155:1341–1351. PMID: 10514416.17. Mavrommatis E, Fish EN, Platanias LC. The schlafen family of proteins and their regulation by interferons. J Interferon Cytokine Res. 2013; 33:206–210. PMID: 23570387.
Article18. Geserick P, Kaiser F, Klemm U, Kaufmann SH, Zerrahn J. Modulation of T cell development and activation by novel members of the Schlafen (slfn) gene family harbouring an RNA helicase-like motif. Int Immunol. 2004; 16:1535–1548. PMID: 15351786.
Article19. Katsoulidis E, Carayol N, Woodard J, Konieczna I, Majchrzak-Kita B, Jordan A, et al. Role of Schlafen 2 (SLFN2) in the generation of interferon α-induced growth inhibitory responses. J Biol Chem. 2009; 284:25051–25064. PMID: 19592487.
Article20. Zhang Y, Song L, Cai L, Wei R, Hu H, Jin W. Effects of baicalein on apoptosis, cell cycle arrest, migration and invasion of osteosarcoma cells. Food Chem Toxicol. 2013; 53:325–333. PMID: 23266503.
Article21. Hsu SL, Hsieh YC, Hsieh WC, Chou CJ. Baicalein induces a dual growth arrest by modulating multiple cell cycle regulatory molecules. Eur J Pharmacol. 2001; 425:165–171. PMID: 11513834.
Article22. Sadaie M, Dillon C, Narita M, Young AR, Cairney CJ, Godwin LS, et al. Cell-based screen for altered nuclear phenotypes reveals senescence progression in polyploid cells after Aurora kinase B inhibition. Mol Biol Cell. 2015; 26:2971–2985. PMID: 26133385.
Article23. Aoshiba K, Tsuji T, Kameyama S, Itoh M, Semba S, Yamaguchi K, et al. Senescence-associated secretory phenotype in a mouse model of bleomycin-induced lung injury. Exp Toxicol Pathol. 2013; 65:1053–1062. PMID: 23688655.
Article24. Lawson WE, Polosukhin VV, Stathopoulos GT, Zoia O, Han W, Lane KB, et al. Increased and prolonged pulmonary fibrosis in surfactant protein C-deficient mice following intratracheal bleomycin. Am J Pathol. 2005; 167:1267–1277. PMID: 16251411.
Article25. Yonezawa R, Yamamoto S, Takenaka M, Kage Y, Negoro T, Toda T, et al. TRPM2 channels in alveolar epithelial cells mediate bleomycin-induced lung inflammation. Free Radic Biol Med. 2016; 90:101–113. PMID: 26600069.
Article26. Kato A, Okura T, Hamada C, Miyoshi S, Katayama H, Higaki J, et al. Cell stress induces upregulation of osteopontin via the ERK pathway in type II alveolar epithelial cells. PLoS One. 2014; 9:e100106. PMID: 24963635.
Article27. Aoshiba K, Tsuji T, Nagai A. Bleomycin induces cellular senescence in alveolar epithelial cells. Eur Respir J. 2003; 22:436–443. PMID: 14516132.
Article28. Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A. 2008; 105:13051–13056. PMID: 18753630.
Article29. Naikawadi RP, Disayabutr S, Mallavia B, Donne ML, Green G, La JL, et al. Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight. 2016; 1:e86704. PMID: 27699234.
Article30. Povedano JM, Martinez P, Flores JM, Mulero F, Blasco MA. Mice with pulmonary fibrosis driven by telomere dysfunction. Cell Rep. 2015; 12:286–299. PMID: 26146081.
Article31. Cui H, Ge J, Xie N, Banerjee S, Zhou Y, Liu RM, et al. miR-34a promotes fibrosis in aged lungs by inducing alveolarepithelial dysfunctions. Am J Physiol Lung Cell Mol Physiol. 2017; 312:L415–L424. PMID: 27979858.
Article32. Brar SS, Meyer JN, Bortner CD, Van Houten B, Martin WJ 2nd. Mitochondrial DNA-depleted A549 cells are resistant to bleomycin. Am J Physiol Lung Cell Mol Physiol. 2012; 303:L413–L424. PMID: 22773697.
Article33. Lee H, Park JR, Kim EJ, Kim WJ, Hong SH, Park SM, et al. Cigarette smoke-mediated oxidative stress induces apoptosis via the MAPKs/STAT1 pathway in mouse lung fibroblasts. Toxicol Lett. 2016; 240:140–148. PMID: 26546778.
Article34. Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998; 282:1497–1501. PMID: 9822382.35. Kang K, Lee SB, Yoo JH, Nho CW. Flow cytometric fluorescence pulse width analysis of etoposide-induced nuclear enlargement in HCT116 cells. Biotechnol Lett. 2010; 32:1045–1052. PMID: 20429026.
Article36. Kaneko M, Matsuda D, Ohtawa M, Fukuda T, Nagamitsu T, Yamori T, et al. Potentiation of bleomycin in Jurkat cells by fungal pycnidione. Biol Pharm Bull. 2012; 35:18–28. PMID: 22223332.
Article37. Shetty SK, Tiwari N, Marudamuthu AS, Puthusseri B, Bhandary YP, Fu J, et al. p53 and miR-34a feedback promotes lung epithelial injury and pulmonary fibrosis. Am J Pathol. 2017; 187:1016–1034. PMID: 28273432.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Heme Oxygenase-1 Induced by Aprotinin Inhibits Vascular Smooth Muscle Cell Proliferation Through Cell Cycle Arrest in Hypertensive Rats
- Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells
- CRM1 inhibitor S109 suppresses cell proliferation and induces cell cycle arrest in renal cancer cells
- Methyl-beta-cyclodextrin inhibits cell growth and cell cycle arrest via a prostaglandin E(2) independent pathway
- C-Type Natriuretic Peptide/Natriuretic Peptide Receptor 2 Is Involved in Cell Proliferation and Testosterone Production in Mouse Leydig Cells