Yonsei Med J.
2019 Jul;60(7):640-650. 10.3349/ymj.2019.60.7.640.
Long Noncoding RNA NEAT1 Aggravates Aβ-Induced Neuronal Damage by Targeting miR-107 in Alzheimer's Disease
- Affiliations
-
- 1Department of Neurology, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, China.
- 2Department of Burn and Plastic Surgery, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, China.
- 3Department of Urology, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, China. liaobonsmc@163.com
- KMID: 2450404
- DOI: http://doi.org/10.3349/ymj.2019.60.7.640
Abstract
- PURPOSE
Alzheimer's disease (AD) is the most common neurodegenerative disease, with a rising prevalence worldwide. Long noncoding RNAs (lncRNAs) have been found to play important roles in the development and treatment of AD. However, the exact role of lncRNA nuclear enriched abundant transcript 1 (NEAT1) in neuronal damage in AD is largely unknown.
MATERIALS AND METHODS
The AD model was established in SH-SY5Y and SK-N-SH cells via treatment with amyloid β1−42 (Aβ). The expression of NEAT1 and microRNA-107 (miR-107) was measured by quantitative real-time polymerase chain reaction. Cell viability and apoptosis were detected by MTT assay, immunocytochemistry, and flow cytometry. The expression of phosphorylated tau protein (p-Tau) was measured by Western blot. The interaction between NEAT1 and miR-107 was explored by bioinformatics analysis, luciferase activity, and RNA immunoprecipitation assays.
RESULTS
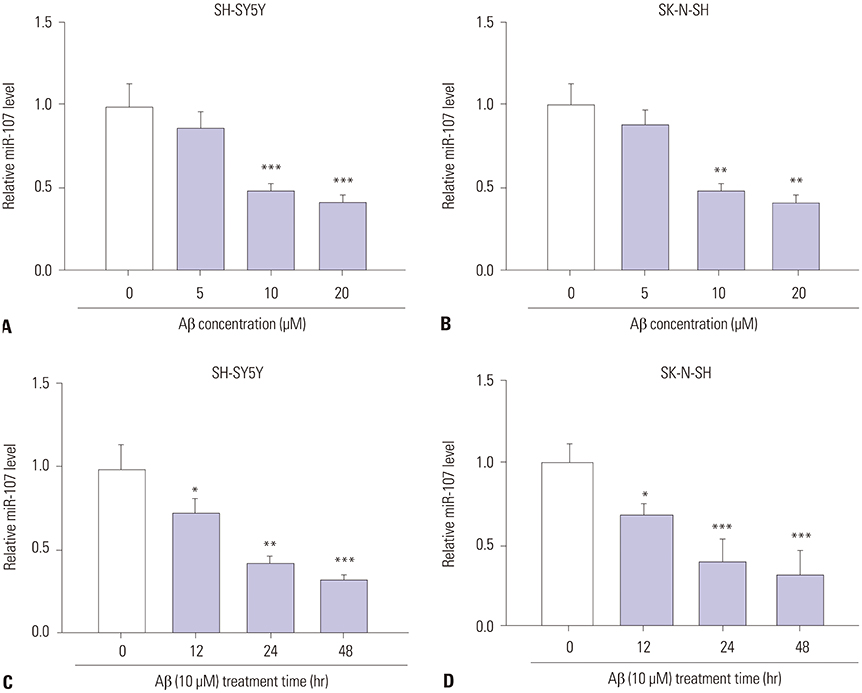
NEAT1 expression was enhanced in Aβ-treated SH-SY5Y and SK-N-SH cells, and its knockdown attenuated Aβ-induced inhibition of viability and promotion of apoptosis and p-Tau levels. NEAT1 was indicated as a decoy of miR-107. miR-107 abundance was reduced in Aβ-treated cells, and its overexpression reversed Aβ-induced injury. Moreover, interference of miR-107 abated silencing of NEAT1-mediated inhibition of neuronal damage in Aβ-treated SH-SY5Y and SK-N-SH cells.
CONCLUSION
LncRNA NEAT1 aggravated Aβ-induced neuronal damage by sponging miR-107, indicating a novel avenue for treatment of AD.
Keyword
MeSH Terms
-
Alzheimer Disease*
Amyloid
Apoptosis
Blotting, Western
Cell Survival
Computational Biology
Flow Cytometry
Immunohistochemistry
Immunoprecipitation
Luciferases
Neurodegenerative Diseases
Neurons*
Prevalence
Real-Time Polymerase Chain Reaction
RNA
RNA, Long Noncoding*
tau Proteins
Amyloid
Luciferases
RNA
RNA, Long Noncoding
tau Proteins
Figure
Reference
-
1. Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, et al. Alzheimer's disease. Lancet. 2016; 388:505–517.
Article2. Chen Y, Fu AKY, Ip NY. Synaptic dysfunction in Alzheimer's disease: mechanisms and therapeutic strategies. Pharmacol Ther. 2019; 195:186–198.
Article3. Nasica-Labouze J, Nguyen PH, Sterpone F, Berthoumieu O, Buchete NV, Coté S, et al. Amyloid β protein and Alzheimer's disease: when computer simulations complement experimental studies. Chem Rev. 2015; 115:3518–3563.
Article4. Li C, Götz J. Tau-based therapies in neurodegeneration: opportunities and challenges. Nat Rev Drug Discov. 2017; 16:863–883.
Article5. Idda ML, Munk R, Abdelmohsen K, Gorospe M. Noncoding RNAs in Alzheimer's disease. Wiley Interdiscip Rev RNA. 2018; 9:e1463.
Article6. Wang DQ, Fu P, Yao C, Zhu LS, Hou TY, Chen JG, et al. Long non-coding RNAs, novel culprits, or bodyguards in neurodegenerative diseases. Mol Ther Nucleic Acids. 2018; 10:269–276.
Article7. Zhang L, Fang Y, Cheng X, Lian YJ, Xu HL. Silencing of long noncoding RNA SOX21-AS1 relieves neuronal oxidative stress injury in mice with Alzheimer's disease by upregulating FZD3/5 via the Wnt signaling pathway. Mol Neurobiol. 2019; 56:3522–3537.
Article8. Gu C, Chen C, Wu R, Dong T, Hu X, Yao Y, et al. Long noncoding RNA EBF3-AS promotes neuron apoptosis in Alzheimer's disease. DNA Cell Biol. 2018; 37:220–226.
Article9. Chanda K, Das S, Chakraborty J, Bucha S, Maitra A, Chatterjee R, et al. Altered levels of long ncRNAs Meg3 and Neat1 in cell and animal models of Huntington's disease. RNA Biol. 2018; 15:1348–1363.
Article10. Yan W, Chen ZY, Chen JQ, Chen HM. LncRNA NEAT1 promotes autophagy in MPTP-induced Parkinson's disease through stabilizing PINK1 protein. Biochem Biophys Res Commun. 2018; 496:1019–1024.
Article11. Liu Y, Lu Z. Long non-coding RNA NEAT1 mediates the toxic of Parkinson's disease induced by MPTP/MPP+ via regulation of gene expression. Clin Exp Pharmacol Physiol. 2018; 45:841–848.
Article12. Spreafico M, Grillo B, Rusconi F, Battaglioli E, Venturin M. Multiple layers of CDK5R1 regulation in Alzheimer's disease implicate long non-coding RNAs. Int J Mol Sci. 2018; 19:2022.
Article13. Martinez B, Peplow PV. MicroRNAs as diagnostic and therapeutic tools for Alzheimer's disease: advances and limitations. Neural Regen Res. 2019; 14:242–255.
Article14. Finnerty JR, Wang WX, Hébert SS, Wilfred BR, Mao G, Nelson PT. The miR-15/107 group of microRNA genes: evolutionary biology, cellular functions, and roles in human diseases. J Mol Biol. 2010; 402:491–509.
Article15. Foley NH, O'Neill LA. miR-107: a toll-like receptor-regulated miRNA dysregulated in obesity and type II diabetes. J Leukoc Biol. 2012; 92:521–527.
Article16. Jiang ZP, Zhou TB. Role of miR-107 and its signaling pathways in diseases. J Recept Signal Transduct Res. 2014; 34:338–341.
Article17. Fransquet PD, Ryan J. Micro RNA as a potential blood-based epigenetic biomarker for Alzheimer's disease. Clin Biochem. 2018; 58:5–14.
Article18. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT Method. Methods. 2001; 25:402–408.
Article19. Jiang Y, Xu B, Chen J, Sui Y, Ren L, Li J, et al. Micro-RNA-137 inhibits tau hyperphosphorylation in Alzheimer's disease and targets the CACNA1C gene in transgenic mice and human neuroblastoma SH-SY5Y cells. Med Sci Monit. 2018; 24:5635–5644.
Article20. Li Q, Li X, Wang L, Zhang Y, Chen L. miR-98-5p acts as a target for Alzheimer's disease by regulating Aβ production through modulating SNX6 expression. J Mol Neurosci. 2016; 60:413–420.
Article21. Dong P, Xiong Y, Yue J, Hanley SJB, Kobayashi N, Todo Y, et al. Long non-coding RNA NEAT1: a novel target for diagnosis and therapy in human tumors. Front Genet. 2018; 9:471.
Article22. Chen DD, Hui LL, Zhang XC, Chang Q. NEAT1 contributes to ox-LDL-induced inflammation and oxidative stress in macrophages through inhibiting miR-128. J Cell Biochem. 2018; 120:2493–2501.
Article23. Zhou K, Zhang C, Yao H, Zhang X, Zhou Y, Che Y, et al. Knockdown of long non-coding RNA NEAT1 inhibits glioma cell migration and invasion via modulation of SOX2 targeted by miR-132. Mol Cancer. 2018; 17:105.
Article24. Zhen Y, Nan Y, Guo S, Zhang L, Li G, Yue S, et al. Knockdown of NEAT1 repressed the malignant progression of glioma through sponging miR-107 and inhibiting CDK14. J Cell Physiol. 2019; 234:10671–10679.
Article25. Yang X, Xiao Z, Du X, Huang L, Du G. Silencing of the long non-coding RNA NEAT1 suppresses glioma stem-like properties through modulation of the miR-107/CDK6 pathway. Oncol Rep. 2017; 37:555–562.
Article26. Wang P, Wu T, Zhou H, Jin Q, He G, Yu H, et al. Long noncoding RNA NEAT1 promotes laryngeal squamous cell cancer through regulating miR-107/CDK6 pathway. J Exp Clin Cancer Res. 2016; 35:22.
Article27. Gupta P, Bhattacharjee S, Sharma AR, Sharma G, Lee SS, Chakraborty C. miRNAs in Alzheimer disease - a therapeutic perspective. Curr Alzheimer Res. 2017; 14:1198–1206.
Article28. Moncini S, Lunghi M, Valmadre A, Grasso M, Del Vescovo V, Riva P, et al. The miR-15/107 family of microRNA genes regulates CDK5R1/p35 with implications for Alzheimer's disease pathogenesis. Mol Neurobiol. 2017; 54:4329–4342.
Article29. Liu W, Cai H, Lin M, Zhu L, Gao L, Zhong R, et al. MicroRNA-107 prevents amyloid-beta induced blood-brain barrier disruption and endothelial cell dysfunction by targeting Endophilin-1. Exp Cell Res. 2016; 343:248–257.
Article30. Wang WX, Rajeev BW, Stromberg AJ, Ren N, Tang G, Huang Q, et al. The expression of microRNA miR-107 decreases early in Alzheimer's disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J Neurosci. 2008; 28:1213–1223.
Article31. Jiao Y, Kong L, Yao Y, Li S, Tao Z, Yan Y, et al. Osthole decreases beta amyloid levels through up-regulation of miR-107 in Alzheimer's disease. Neuropharmacology. 2016; 108:332–344.
Article32. Shu B, Zhang X, Du G, Fu Q, Huang L. MicroRNA-107 prevents amyloid-β-induced neurotoxicity and memory impairment in mice. Int J Mol Med. 2018; 41:1665–1672.
Article33. Götz J, Bodea LG, Goedert M. Rodent models for Alzheimer disease. Nat Rev Neurosci. 2018; 19:583–598.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Knockdown of Long Non-Coding RNA NEAT1 Inhibits Proliferation and Invasion and Induces Apoptosis of Osteosarcoma by Inhibiting miR-194 Expression
- The Long Noncoding RNA NEAT1 Targets miR-34a-5p and Drives Nasopharyngeal Carcinoma Progression via Wnt/β-Catenin Signaling
- Paper “Inhibition of Long Noncoding RNA SNHG15 Ameliorates Hypoxia/Ischemia-Induced Neuronal Damage by Regulating miR-302a-3p/STAT1/NF-κB Axis” by Hu C, et al.[Yonsei Med J 2021;62(4):325-337]
- Long Noncoding RNA FBXL19-AS1-Mediated Ulcerative Colitis-Associated Intestinal Epithelial Barrier Defect
- Long Non-Coding RNA LINC00525 Promotes the Stemness and Chemoresistance of Colorectal Cancer by Targeting miR-507/ELK3 Axis