Nutr Res Pract.
2018 Dec;12(6):503-511. 10.4162/nrp.2018.12.6.503.
The antioxidant activity of steamed ginger and its protective effects on obesity induced by high-fat diet in C57BL/6J mice
- Affiliations
-
- 1Department of Food Science and Human Nutrition, Chonbuk National University and Obesity Research Center, 567 Baekje-daero, Dukjin-gu, Jeonju, Jeonbuk 54896, Korea. cha8@jbnu.ac.kr
- 2Department of Food Science and Nutrition, Pusan National University, Busan 46241, Korea.
- 3Healthy Local Food Branding Agency, Jeonbuk, 55310, Korea.
- KMID: 2448695
- DOI: http://doi.org/10.4162/nrp.2018.12.6.503
Abstract
- BACKGROUND/OBJECTIVES
Ginger, a root vegetable, is known to have antioxidant and antiobesity effects. Preparation, such as by steaming, can affect the chemical composition of prepared root vegetables or herbs and can change their functional activities. In the present study, we investigated the protective effects of steamed ginger against oxidative stress and steatosis in C57BL/6J mice fed a high-fat diet.
MATERIALS/METHODS
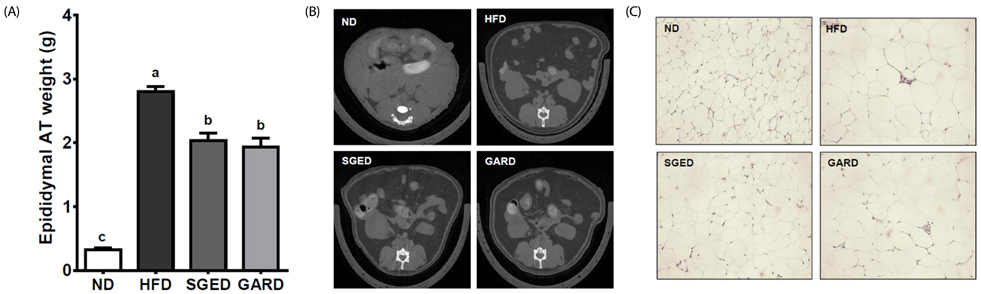
The levels of polyphenols and flavonoids in two different extracts of steamed ginger, i.e., water extract (SGW) and ethanolic extract (SGE); as well, their antioxidant activities were examined. Forty male C57BL/6J mice were fed a normal diet (ND, n = 10), high-fat diet (HFD, 60% fat, w/w, n = 10), HFD supplemented with 200 mg/kg of SGE or garcinia (GAR) by weight (SGED or GARD, respectively, n = 10) for 12 weeks. Serum chemistry was examined, and the expressions of genes involved in lipid metabolism were determined in the liver. Histological analysis was performed to identify lipid accumulations in epididymal fat pads and liver.
RESULTS
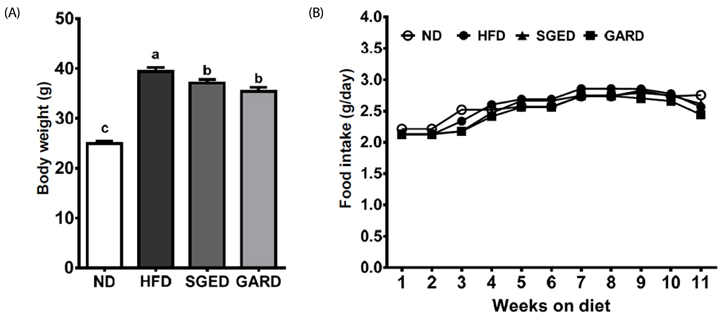
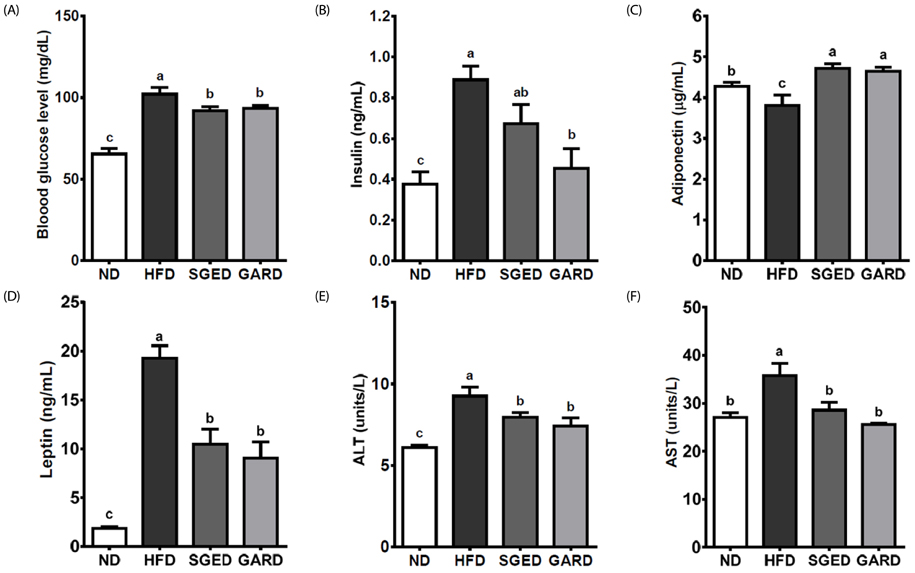
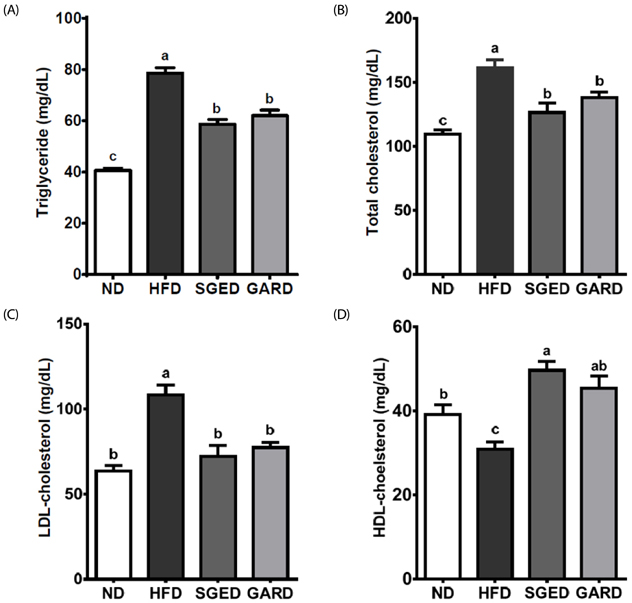
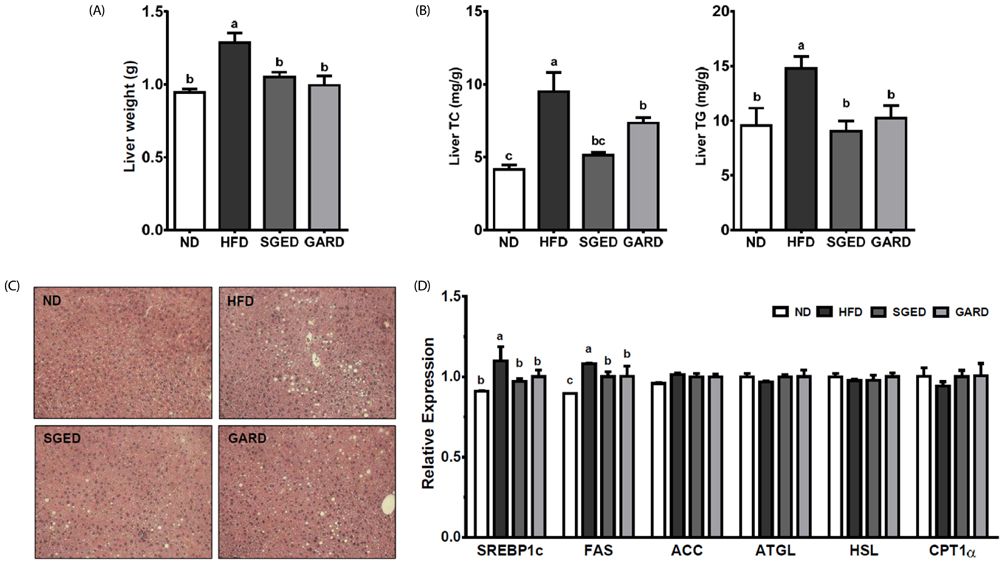
The SGE had higher contents of polyphenols and flavonoids and higher DPPH and ABTS⺠free radical scavenging activities compared to those of SGW. Treatment with SGE or GAR significantly decreased the HFD-induced weight gain. Both SGE and GAR significantly reduced the high serum total cholesterol (TC), triglyceride (TG) and low-density lipoprotein levels induced by HFD. Compared to ND, HFD significantly increased hepatic TC and TG levels. SGE or GAR supplementation significantly decreased the increase of hepatic lipids by HFD. Interestingly, SGE had a more significant effect in reducing hepatic TC and TG levels than GAR. Furthermore, hepatic genes involved in lipogenesis and lipolysis were altered in both the SGED and GARD groups.
CONCLUSIONS
The present study indicates that steamed ginger supplementation can decrease plasma TC and TG and can inhibit liver steatosis by regulating the expressions of hepatic genes.
MeSH Terms
-
Adipose Tissue
Animals
Chemistry
Cholesterol
Diet
Diet, High-Fat*
Ethanol
Fatty Liver
Flavonoids
Garcinia
Ginger*
Humans
Lipid Metabolism
Lipogenesis
Lipolysis
Lipoproteins
Liver
Male
Mice*
Obesity*
Oxidative Stress
Plasma
Polyphenols
Steam*
Triglycerides
Vegetables
Water
Weight Gain
Cholesterol
Ethanol
Flavonoids
Lipoproteins
Polyphenols
Steam
Water
Figure
Cited by 2 articles
-
Antioxidant and antiobesity activities of oral treatment with ethanol extract from sprout of evening primrose (Oenothera laciniata) in high fat diet-induced obese mice
Chung Shil Kwak, Mi-Ju Kim, Sun Gi Kim, Sunyeong Park, In Gyu Kim, Heun Soo Kang
J Nutr Health. 2019;52(6):529-539. doi: 10.4163/jnh.2019.52.6.529.The protective effects of steamed ginger on adipogenesis in 3T3-L1 cells and adiposity in diet-induced obese mice
Bohkyung Kim, Hee-Jeong Kim, Youn-Soo Cha
Nutr Res Pract. 2021;15(3):279-293. doi: 10.4162/nrp.2021.15.3.279.
Reference
-
1. Botchlett R, Woo SL, Liu M, Pei Y, Guo X, Li H, Wu CDC. Nutritional approaches for managing obesity-associated metabolic diseases. J Endocrinol. 2017; 233:R145–R171.
Article2. Abdali D, Samson SE, Grover AK. How effective are antioxidant supplements in obesity and diabetes? Med Princ Pract. 2015; 24:201–215.
Article3. Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013; 7:e330–e341.
Article4. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004; 114:1752–1761.
Article5. Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem. 2015; 97:55–74.
Article6. Chrubasik S, Pittler MH, Roufogalis BD. Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine. 2005; 12:684–701.
Article7. Haniadka R, Saldanha E, Sunita V, Palatty PL, Fayad R, Baliga MS. A review of the gastroprotective effects of ginger (Zingiber officinale Roscoe). Food Funct. 2013; 4:845–855.
Article8. Marx W, McKavanagh D, McCarthy AL, Bird R, Ried K, Chan A, Isenring L. The effect of ginger (Zingiber officinale) on platelet aggregation: a systematic literature review. PLoS One. 2015; 10:e0141119.
Article9. Tohma H, Gulcin I, Bursal E, Goren AC, Alwasel SH, Koksal E. Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc.) determined by HPLC-MS/MS. J Food Meas Charact. 2017; 11:556–566.
Article10. Sakulnarmrat K, Srzednicki G, Konczak I. Antioxidant, enzyme inhibitory and antiproliferative activity of polyphenolic-rich fraction of commercial dry ginger powder. Int J Food Sci Technol. 2015; 50:2229–2235.
Article11. Jelled A, Fernandes A, Barros L, Chahdoura H, Achour L, Ferreira IC, Ben Cheikh H. Chemical and antioxidant parameters of dried forms of ginger rhizomes. Ind Crops Prod. 2015; 77:30–35.
Article12. Guo J, Wu H, Du L, Zhang W, Yang J. Comparative antioxidant properties of some gingerols and shogaols, and the relationship of their contents with the antioxidant potencies of fresh and dried ginger (Zingiber officinale Roscoe). J Agr Sci Tech. 2014; 16:1063–1072.13. Shi CJ, Wen XS, Gao HF, Liu ZH, Xu XK, Li LF, Shen T, Xian CJ. Steamed root of Rehmannia glutinosa Libosch (Plantaginaceae) alleviates methotrexate-induced intestinal mucositis in rats. J Ethnopharmacol. 2016; 183:143–150.
Article14. Jun HI, Yang JH, Choi JY, Lee SH, Song GS, Kim KS, Kim YS. Changes in volatile flavor compounds in steam-dried Allium hookeri root. Food Sci Biotechnol. 2016; 25:1327–1331.
Article15. Gu CZ, Lv JJ, Zhang XX, Yan H, Zhu HT, Luo HR, Wang D, Yang CR, Xu M, Zhang YJ. Minor dehydrogenated and cleavaged dammarane-type saponins from the steamed roots of Panax notoginseng. Fitoterapia. 2015; 103:97–105.
Article16. Gu CZ, Lv JJ, Zhang XX, Qiao YJ, Yan H, Li Y, Wang D, Zhu HT, Luo HR, Yang CR, Xu M, Zhang YJ. Triterpenoids with promoting effects on the differentiation of PC12 cells from the steamed roots of panax notoginseng. J Nat Prod. 2015; 78:1829–1840.
Article17. Nakamura Y, Kuranouchi T, Ohara-Takada A, Katayama K. The effects of β-amylase activity and starch pasting temperature on maltose generation in steamed storage roots of sweet potato. J Jpn Soc Food Sci. 2014; 61:577–585.
Article18. Fletes-Arjona VM, Soto-Dominguez A, Garcia-Garza R, Moran-Martinez J, Benitez-Valle C, Castaneda-Martinez A, Montalvo-Gonzalez R, Becerra-Verdin EM. Morphologic alterations in the respiratory tract of wistar rats induced by steams of the root of Hierba del Zorrillo (Petiveria alliacea) from southwest of Mexico. Int J Morphol. 2013; 31:121–127.
Article19. Zhang J, Yu K, Millar A, Gao Y, Ma B, Kang L, Yu H. Comparison of extracts from dry and alcohol-steamed root of Polygonatum kingianum (Huang Jing) by sub-2-μm-LC-TOF-MS. Curr Trends Mass Spectrom. 2011; 9:30–35.20. Liu Z, Liu Y, Chao Z, Song Z, Wang C, Lu A. In vitro antioxidant activities of maillard reaction products produced in the steaming process of Polygonum multiflorum root. Nat Prod Commun. 2011; 6:55–58.
Article21. Sun S, Wang CZ, Tong R, Li XL, Fishbein A, Wang Q, He TC, Du W, Yuan CS. Effects of steaming the root of Panax notoginseng on chemical composition and anticancer activities. Food Chem. 2010; 118:307–314.
Article22. Arjona CA, Rodriguez-Fragoso L, Rosado-Vallado M, Fitz-Aranda C, Mendoza-Rivera B, Reyes-Esparza J. Pharmacological and toxicological evaluation of methanolic extracts from leaves, steam and roots of a Cnidoscolus plant. FASEB J. 2010; 24.
Article23. Turkmen N, Sari F, Velioglu YS. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chem. 2006; 99:835–841.
Article24. Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999; 64:555–559.
Article25. Mau JL, Lin HC, Song SF. Antioxidant properties of several specialty mushrooms. Food Res Int. 2002; 35:519–526.
Article26. Hatano T, Edamatsu R, Hiramatsu M, Mori A, Fujita Y, Yasuhara T, Yoshida T, Okuda T. Effects of the interaction of tannins with co-existing substances. 6. Effects of tannins and related polyphenols on superoxide anion radical, and on 1,1-diphenyl-2-picrylhydrazyl radical. Chem Pharm Bull (Tokyo). 1989; 37:2016–2021.
Article27. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999; 26:1231–1237.
Article28. Thomas SS, Kim M, Lee SJ, Cha YS. Antiobesity effects of purple perilla (Perilla frutescens var. acuta) on adipocyte differentiation and mice fed a high-fat Diet. J Food Sci. 2018; 83:2384–2393.
Article29. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499–502.
Article30. Kim M, Park JE, Song SB, Cha YS. Effects of black adzuki bean (Vigna angularis) extract on proliferation and differentiation of 3T3-L1 preadipocytes into mature adipocytes. Nutrients. 2015; 7:277–292.
Article31. Meigs JB, Wilson PW, Fox , Vasan RS, Nathan DM, Sullivan LM, D'Agostino RB. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006; 91:2096–2012.
Article32. Keller KB, Lemberg L. Obesity and the metaboic syndrome. Am J Crit Care. 2003; 12:167–170.33. Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González A, Esquivel-Chirino C, Durante-Montiel I, Sánchez-Rivera G, Valadez-Vega C, Morales-González JA. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011; 12:3117–3132.
Article34. Bondia-Pons I, Ryan L. Martinez JA. Oxidative stress and inflammation interactions in human obesity. J Physiol Biochem. 2012; 68:701–711.
Article35. Galassetti P. Inflammation and oxidative stress in obesity, metabolic syndrome, and diabetes. Exp Diabetes Res. 2012; 2012:943706.
Article36. Marseglia L, Manti S, D'Angelo G, Nicotera A, Parisi E, Di Rosa G, Gitto E, Arrigo T. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci. 2014; 16:378–400.37. Valdecantos MP, Pérez-Matute P, Martínez JA. Obesity and oxidative stress: role of antioxidant supplementation. Rev Invest Clin. 2009; 61:127–139.38. Wang J, Ke W, Bao R, Hu X, Chen F. Beneficial effects of ginger Zingiber officinale Roscoe on obesity and metabolic syndrome: a review. Ann N Y Acad Sci. 2017; 1398:83–98.
Article39. Lohachoompol V, Srzednicki G, Craske J. The change of total anthocyanins in blueberries and their antioxidant effect after drying and freezing. J Biomed Biotechnol. 2004; 2004:248–252.
Article40. ElRokh el SM, Yassin NA, El-Shenawy SM, Ibrahim BM. Antihypercholesterolaemic effect of ginger rhizome (Zingiber officinale) in rats. Inflammopharmacology. 2010; 18:309–315.
Article41. Khosravani M, Azarbayjani MA, Abolmaesoomi M, Yusof A, Abidin NZ, Rahimi E, Feizolahi F, Akbari M, Seyedjalali S, Dehghan F. Ginger extract and aerobic training reduces lipid profile in high-fat fed diet rats. Eur Rev Med Pharmacol Sci. 2016; 20:1617–1622.42. Bhandari U, Sharma JN, Zafar R. The protective action of ethanolic ginger (Zingiber officinale) extract in cholesterol fed rabbits. J Ethnopharmacol. 1998; 61:167–171.
Article43. Bhandari U, Kanojia R, Pillai KK. Effect of ethanolic extract of Zingiber officinale on dyslipidaemia in diabetic rats. J Ethnopharmacol. 2005; 97:227–230.
Article44. Nammi S, Sreemantula S, Roufogalis BD. Protective effects of ethanolic extract of Zingiber officinale rhizome on the development of metabolic syndrome in high-fat diet-fed rats. Basic Clin Pharmacol Toxicol. 2009; 104:366–373.
Article45. de Las Heras N, Valero-Muñoz M, Martín-Fernández B, Ballesteros S, López-Farré A, Ruiz-Roso B, Lahera V. Molecular factors involved in the hypolipidemic- and insulin-sensitizing effects of a ginger (Zingiber officinale Roscoe) extract in rats fed a high-fat diet. Appl Physiol Nutr Metab. 2017; 42:209–215.
Article46. Lei L, Liu Y, Wang X, Jiao R, Ma KY, Li YM, Wang L, Man SW, Sang S, Huang Y, Chen ZY. Plasma cholesterol-lowering activity of gingerol- and shogaol-enriched extract is mediated by increasing sterol excretion. J Agric Food Chem. 2014; 62:10515–10521.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The protective effects of steamed ginger on adipogenesis in 3T3-L1 cells and adiposity in diet-induced obese mice
- Erratum: The antioxidant activity of steamed ginger and its protective effects on obesity induced by high-fat diet in C57BL/6J mice
- Effects of fermented blueberry liquid in high-fat diet-induced obese C57BL/6J mice
- The protective effects of Aster yomena (Kitam.) Honda on high-fat diet-induced obese C57BL/6J mice
- Red beet (Beta vulgaris L.) leaf supplementation improves antioxidant status in C57BL/6J mice fed high fat high cholesterol diet