Clin Exp Otorhinolaryngol.
2018 Dec;11(4):242-249. 10.21053/ceo.2017.01641.
Urine Cotinine Should Be Involved in Initial Evaluation of Tinnitus in Adolescents
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, SMG-SNU Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea. yhkiment@gmail.com
- KMID: 2439850
- DOI: http://doi.org/10.21053/ceo.2017.01641
Abstract
OBJECTIVES
Smoking is associated with hearing loss, while the correlation between tinnitus and smoking is not fully elucidated. This study aimed to evaluate risk factors of tinnitus in adolescents in terms of smoking, and we identified a rectifiable parameter that can be serially monitored.
METHODS
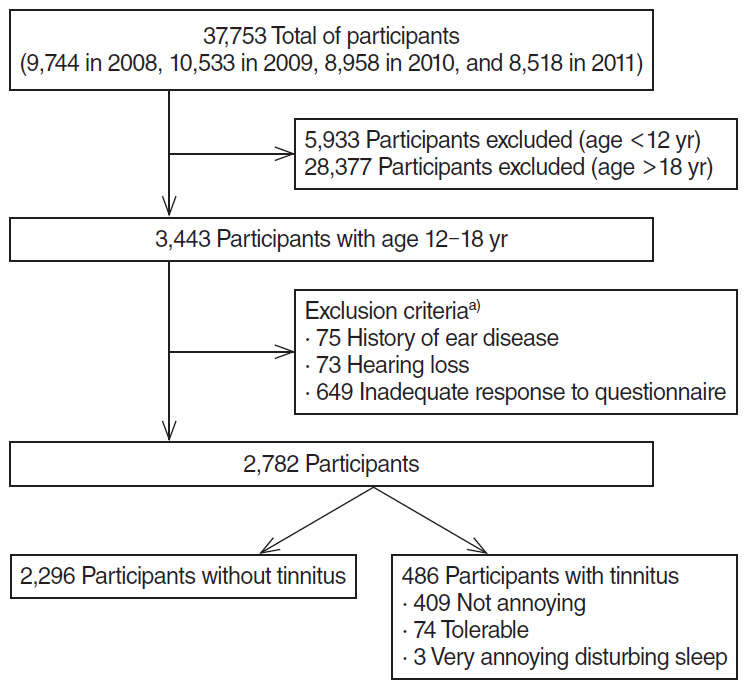
A cross-sectional study was conducted using data from the Korea National Health and Nutrition Examination Survey, with 2,782 participants aged 12 to 18 years, from 2008 through 2011. Participants with history of ear disease, hearing loss, and inadequate responses to questionnaires were excluded. We investigated the prevalence of tinnitus and tinnitus-related annoyance by questionnaire and sought potential risk factors in blood and urine tests and smoking history.
RESULTS
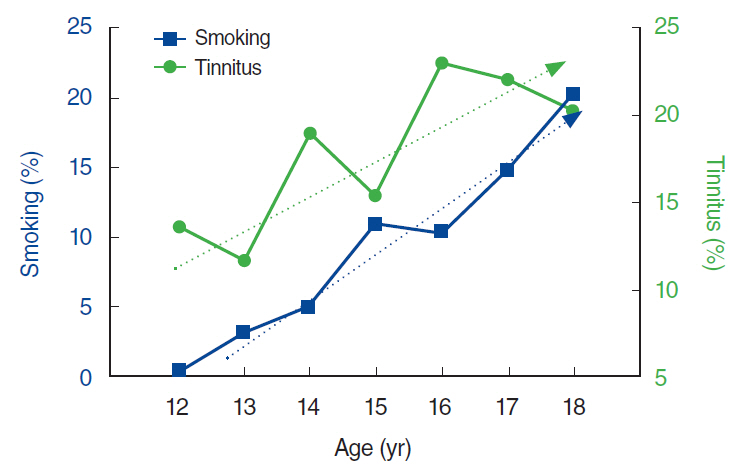
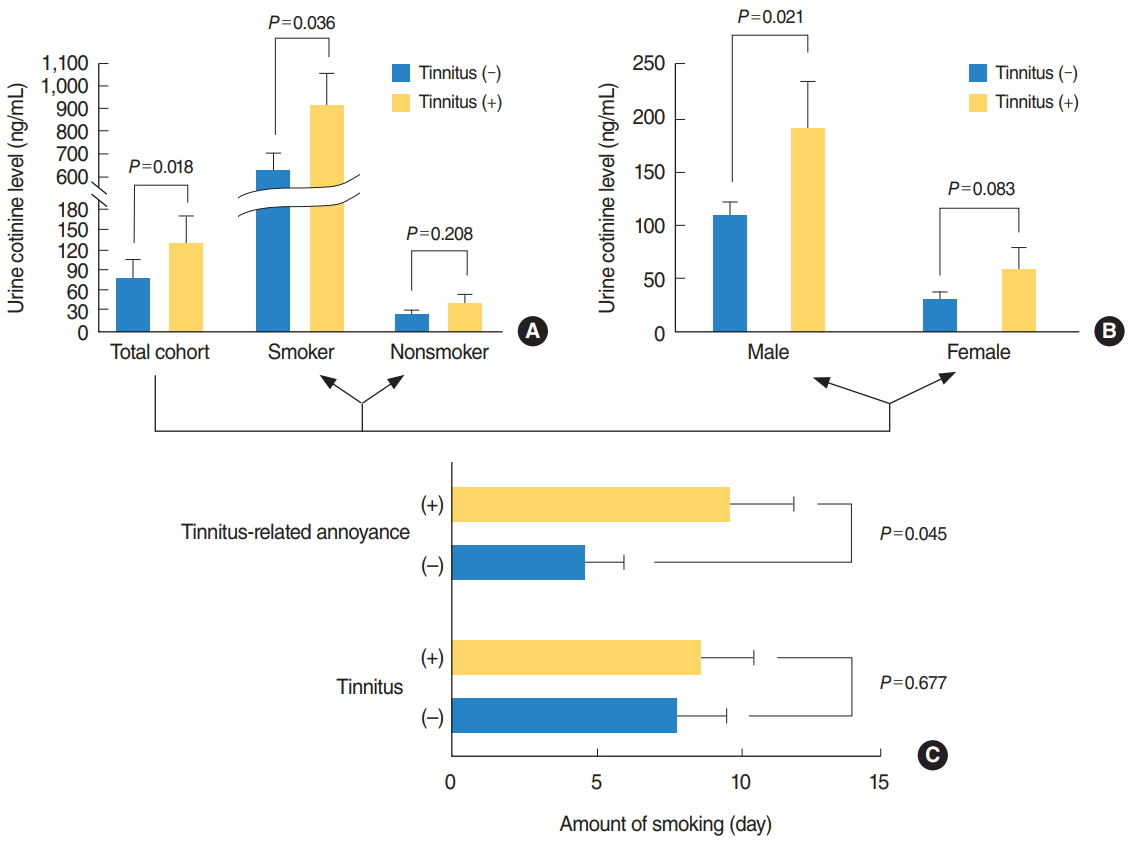
The prevalence of tinnitus in the 12- to 18-year-old population was 17.5%, with 3.3% reporting tinnitus-related annoyance. On univariate analysis, the prevalence of tinnitus increased with age (P < 0.001) and was higher among girls (P=0.012). Blood tests and urinalysis showed significant correlation between tinnitus and red blood cell count, alkaline phosphatase levels, and urine cotinine (P=0.002, P < 0.001, P=0.018, respectively). In multivariate analysis, the urine cotinine level was the only parameter associated with tinnitus (odds ratio, 1.000; 95% confidence interval, 0.999 to 1.000; P=0.038). Smoking was also significantly correlated with tinnitus (P=0.043), and amount of smoking with tinnitus-related annoyance (P=0.045). However, current smoking and past smoking were not correlated with tinnitus.
CONCLUSION
Urine cotinine may be a rectifiable marker for management of tinnitus in adolescents. This suggests that smoking cessation should be incorporated in the management of tinnitus in adolescents.
MeSH Terms
Figure
Reference
-
1. Savastano M, Marioni G, de Filippis C. Tinnitus in children without hearing impairment. Int J Pediatr Otorhinolaryngol. 2009; Dec. 73 Suppl 1:S13–5.
Article2. Aust G. Tinnitus in childhood. Int Tinnitus J. 2002; 8(1):20–6.3. Savastano M. Characteristics of tinnitus in childhood. Eur J Pediatr. 2007; Aug. 166(8):797–801.
Article4. Mahboubi H, Oliaei S, Kiumehr S, Dwabe S, Djalilian HR. The prevalence and characteristics of tinnitus in the youth population of the United States. Laryngoscope. 2013; Aug. 123(8):2001–8.
Article5. Park B, Choi HG, Lee HJ, An SY, Kim SW, Lee JS, et al. Analysis of the prevalence of and risk factors for tinnitus in a young population. Otol Neurotol. 2014; Aug. 35(7):1218–22.
Article6. Kim HJ, Lee HJ, An SY, Sim S, Park B, Kim SW, et al. Analysis of the prevalence and associated risk factors of tinnitus in adults. PLoS One. 2015; May. 10(5):e0127578.
Article7. Gallus S, Lugo A, Garavello W, Bosetti C, Santoro E, Colombo P, et al. Prevalence and determinants of tinnitus in the Italian adult population. Neuroepidemiology. 2015; Aug. 45(1):12–9.
Article8. Kim YH, Jung HJ, Kang SI, Park KT, Choi JS, Oh SH, et al. Tinnitus in children: association with stress and trait anxiety. Laryngoscope. 2012; Oct. 122(10):2279–84.
Article9. Coelho CB, Sanchez TG, Tyler RS. Tinnitus in children and associated risk factors. Prog Brain Res. 2007; Oct. 166:179–91.
Article10. Henderson E, Testa MA, Hartnick C. Prevalence of noise-induced hearing-threshold shifts and hearing loss among US youths. Pediatrics. 2011; Jan. 127(1):e39–46.
Article11. Niskar AS, Kieszak SM, Holmes AE, Esteban E, Rubin C, Brody DJ. Estimated prevalence of noise-induced hearing threshold shifts among children 6 to 19 years of age: the Third National Health and Nutrition Examination Survey, 1988-1994, United States. Pediatrics. 2001; Jul. 108(1):40–3.
Article12. Bulbul SF, Muluk NB, Cakir EP, Tufan E. Subjective tinnitus and hearing problems in adolescents. Int J Pediatr Otorhinolaryngol. 2009; Aug. 73(8):1124–31.
Article13. Brunnberg E, Linden-Bostrom M, Berglund M. Tinnitus and hearing loss in 15-16-year-old students: mental health symptoms, substance use, and exposure in school. Int J Audiol. 2008; Nov. 47(11):688–94.
Article14. Lantz PM. Smoking on the rise among young adults: implications for research and policy. Tob Control. 2003; Jun. 12 Suppl 1:i60–70.
Article15. Nelson DE, Mowery P, Asman K, Pederson LL, O’Malley PM, Malarcher A, et al. Long-term trends in adolescent and young adult smoking in the United States: metapatterns and implications. Am J Public Health. 2008; May. 98(5):905–15.
Article16. Best CM, Sun K, de Pee S, Bloem MW, Stallkamp G, Semba RD. Parental tobacco use is associated with increased risk of child malnutrition in Bangladesh. Nutrition. 2007; Oct. 23(10):731–8.
Article17. Sharabi Y, Reshef-Haran I, Burstein M, Eldad A. Cigarette smoking and hearing loss: lessons from the young adult periodic examinations in Israel (YAPEIS) database. Isr Med Assoc J. 2002; Dec. 4(12):1118–20.18. Agius AM, Wake M, Pahor AL, Smallman LA. Smoking and middle ear ciliary beat frequency in otitis media with effusion. Acta Otolaryngol. 1995; Jan. 115(1):44–9.
Article19. Fransen E, Topsakal V, Hendrickx JJ, Van Laer L, Huyghe JR, Van Eyken E, et al. Occupational noise, smoking, and a high body mass index are risk factors for age-related hearing impairment and moderate alcohol consumption is protective: a European population-based multicenter study. J Assoc Res Otolaryngol. 2008; Sep. 9(3):264–76.
Article20. Cruickshanks KJ, Klein R, Klein BE, Wiley TL, Nondahl DM, Tweed TS. Cigarette smoking and hearing loss: the epidemiology of hearing loss study. JAMA. 1998; Jun. 279(21):1715–9.21. Martines F, Sireci F, Cannizzaro E, Costanzo R, Martines E, Mucia M, et al. Clinical observations and risk factors for tinnitus in a Sicilian cohort. Eur Arch Otorhinolaryngol. 2015; Oct. 272(10):2719–29.
Article22. Chang J, Ryou N, Jun HJ, Hwang SY, Song JJ, Chae SW. Effect of cigarette smoking and passive smoking on hearing impairment: data from a population-based study. PLoS One. 2016; Jan. 11(1):e0146608.
Article23. Langguth B. Treatment of tinnitus. Curr Opin Otolaryngol Head Neck Surg. 2015; Oct. 23(5):361–8.24. Yang S, Weiner BD, Zhang LS, Cho SJ, Bao S. Homeostatic plasticity drives tinnitus perception in an animal model. Proc Natl Acad Sci U S A. 2011; Sep. 108(36):14974–9.
Article25. Friberg E, Jansson C, Mittendorfer-Rutz E, Rosenhall U, Alexanderson K. Sickness absence due to otoaudiological diagnoses and risk of disability pension: a nationwide Swedish prospective cohort study. PLoS One. 2012; 7(1):e29966.
Article26. Lowe GD, Drummond MM, Forbes CD, Barbenel JC. The effects of age and cigarette-smoking on blood and plasma viscosity in men. Scott Med J. 1980; Jan. 25(1):13–7.
Article27. Negley C, Katbamna B, Crumpton T, Lawson GD. Effects of cigarette smoking on distortion product otoacoustic emissions. J Am Acad Audiol. 2007; Sep. 18(8):665–74.
Article28. Torre P 3rd, Dreisbach LE, Kopke R, Jackson R, Balough B. Risk factors for distortion product otoacoustic emissions in young men with normal hearing. J Am Acad Audiol. 2007; Oct. 18(9):749–59.
Article29. Elgayar SA, Hussein OA, Abdel-Hafez AM, Thabet HS. Nicotine impact on the structure of adult male guinea pig auditory cortex. Exp Toxicol Pathol. 2016; Feb-Mar. 68(2-3):167–79.
Article30. Liang K, Poytress BS, Chen Y, Leslie FM, Weinberger NM, Metherate R. Neonatal nicotine exposure impairs nicotinic enhancement of central auditory processing and auditory learning in adult rats. Eur J Neurosci. 2006; Aug. 24(3):857–66.
Article31. Levine H, Berman T, Goldsmith R, Goen T, Spungen J, Novack L, et al. Exposure to tobacco smoke based on urinary cotinine levels among Israeli smoking and nonsmoking adults: a cross-sectional analysis of the first Israeli human biomonitoring study. BMC Public Health. 2013; Dec. 13:1241.
Article32. Crinnion WJ. The CDC fourth national report on human exposure to environmental chemicals: what it tells us about our toxic burden and how it assist environmental medicine physicians. Altern Med Rev. 2010; Jul. 15(2):101–9.33. Matsumoto A, Matsumoto A, Ichiba M, Payton NM, Oishi H, Hara M. Simultaneous measurement of urinary total nicotine and cotinine as biomarkers of active and passive smoking among Japanese individuals. Environ Health Prev Med. 2013; May. 18(3):244–50.
Article34. Jones-Burton C, Vessal G, Brown J, Dowling TC, Fink JC. Urinary cotinine as an objective measure of cigarette smoking in chronic kidney disease. Nephrol Dial Transplant. 2007; Jul. 22(7):1950–4.
Article35. Wang Y, Yang M, Tian L, Huang Z, Chen F, Hu J, et al. Relationship between caregivers’ smoking at home and urinary levels of cotinine in children. Int J Environ Res Public Health. 2014; Dec. 11(12):12499–513.
Article36. Lu L, Mackay DF, Newby DE, Pell JP. Association between salivary cotinine and cardiovascular biomarkers among nonsmokers and current smokers: cross-sectional study of 10,081 participants. Eur J Vasc Endovasc Surg. 2014; Dec. 48(6):703–10.
Article37. Zierk J, Arzideh F, Haeckel R, Cario H, Fruhwald MC, Grob HJ, et al. Pediatric reference intervals for alkaline phosphatase. Clin Chem Lab Med. 2017; Jan. 55(1):102–10.
Article38. Fischer DC, Mischek A, Wolf S, Rahn A, Salweski B, Kundt G, et al. Paediatric reference values for the C-terminal fragment of fibroblast-growth factor-23, sclerostin, bone-specific alkaline phosphatase and isoform 5b of tartrate-resistant acid phosphatase. Ann Clin Biochem. 2012; Nov. 49(Pt 6):546–53.
Article39. Erlandsson SI, Holgers KM. The impact of perceived tinnitus severity on health-related quality of life with aspects of gender. Noise Health. 2001; 3(10):39–51.40. Nater UM, Abbruzzese E, Krebs M, Ehlert U. Sex differences in emotional and psychophysiological responses to musical stimuli. Int J Psychophysiol. 2006; Nov. 62(2):300–8.
Article41. Vanneste S, Joos K, De Ridder D. Prefrontal cortex based sex differences in tinnitus perception: same tinnitus intensity, same tinnitus distress, different mood. PLoS One. 2012; 7(2):e31182.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Urine Cotinine and Environmental Tobacco Exposure in Korean Adolescents
- Smoking Prevalence and Factors Associated with False Reporting in Korean Adolescents: The Korea National Health and Nutrition Examination Survey (2016–2020)
- A Study of the Relationship between Adolescent's Self Reported Cigarette Smoking and Urine Cotinine Level
- Urine Cotinine and Environmental Tobacco Exposure in Korean Adolescents
- Agreement between Smoking Self-report and Urine Cotinine among Adolescents