Ann Dermatol.
2012 May;24(2):126-135.
Cathelicidin LL-37: An Antimicrobial Peptide with a Role in Inflammatory Skin Disease
- Affiliations
-
- 1Department of Dermatology and Allergy, Ludwig-Maximilian-University, Munich, Germany. juergen.schauber@med.uni-muenchen.de
Abstract
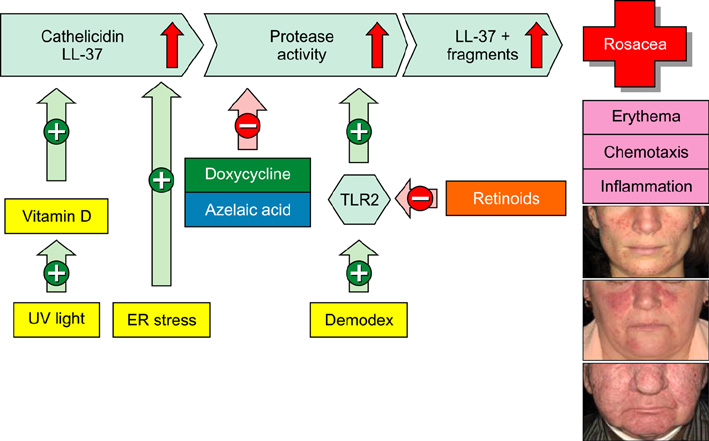
- Chronic inflammatory skin diseases such as atopic dermatitis, psoriasis or rosacea are very common. Although their exact pathogenesis is not completely understood all three diseases are characterized by dysregulation of cutaneous innate immunity. Cathelicidin LL-37 is an important effector molecule of innate immunity in the skin and atopic dermatitis, psoriasis or rosacea show defects in cathelicidin expression, function or processing. In atopic dermatitis, cathelicidin induction might be disturbed resulting in defective antimicrobial barrier function. In contrast, psoriasis is characterized by overexpression of cathelicidin. However to date it is unclear whether pro- or anti-inflammatory functions of cathelicidin predominate in lesional skin in psoriasis. In rosacea, cathelicidin processing is disturbed resulting in peptide fragments causing inflammation, erythema and telangiectasias. In this review, the current evidence on the role of cathelicidin LL-37 in the pathogenesis of inflammatory skin diseases will be outlined. As cathelicidin LL-37 might also serve as a future treatment target potential novel treatment strategies for those diseases will be discussed.
MeSH Terms
Figure
Reference
-
1. Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009. 9:679–691.
Article2. Pasupuleti M, Schmidtchen A, Malmsten M. Antimicrobial peptides: key components of the innate immune system. Crit Rev Biotechnol. 2011. [Epub ahead of print].
Article3. Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003. 55:27–55.
Article4. Schauber J, Gallo RL. Expanding the roles of antimicrobial peptides in skin: alarming and arming keratinocytes. J Invest Dermatol. 2007. 127:510–512.
Article5. Braff MH, Zaiou M, Fierer J, Nizet V, Gallo RL. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun. 2005. 73:6771–6781.
Article6. Murakami M, Ohtake T, Dorschner RA, Schittek B, Garbe C, Gallo RL. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J Invest Dermatol. 2002. 119:1090–1095.
Article7. Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001. 117:91–97.
Article8. Antal AS, Dombrowski Y, Koglin S, Ruzicka T, Schauber J. Impact of vitamin D3 on cutaneous immunity and antimicrobial peptide expression. Dermatoendocrinol. 2011. 3:18–22.
Article9. Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol. 2006. 306:91–110.
Article10. Zhang G, Ross CR, Blecha F. Porcine antimicrobial peptides: new prospects for ancient molecules of host defense. Vet Res. 2000. 31:277–296.
Article11. Wang Z, Wang G. APD: the antimicrobial peptide database. Nucleic Acids Res. 2004. 32(Database issue):D590–D592.
Article12. Peric M, Koglin S, Kim SM, Morizane S, Besch R, Prinz JC, et al. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J Immunol. 2008. 181:8504–8512.
Article13. Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001. 276:5707–5713.
Article14. Schauber J, Dorschner RA, Coda AB, Büchau AS, Liu PT, Kiken D, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007. 117:803–811. Epub 2007 Feb 8.
Article15. Wolk K, Witte E, Wallace E, Döcke WD, Kunz S, Asadullah K, et al. L-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006. 36:1309–1323.
Article16. Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006. 20:2068–2080.
Article17. Michaelson D, Rayner J, Couto M, Ganz T. Cationic defensins arise from charge-neutralized propeptides: a mechanism for avoiding leukocyte autocytotoxicity? J Leukoc Biol. 1992. 51:634–639.
Article18. Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005. 6:551–557.
Article19. Zaiou M, Gallo RL. Cathelicidins, essential gene-encoded mammalian antibiotics. J Mol Med (Berl). 2002. 80:549–561.
Article20. Harder J, Siebert R, Zhang Y, Matthiesen P, Christophers E, Schlegelberger B, et al. Mapping of the gene encoding human beta-defensin-2 (DEFB2) to chromosome region 8p22-p23.1. Genomics. 1997. 46:472–475.
Article21. Schröder JM, Harder J. Human beta-defensin-2. Int J Biochem Cell Biol. 1999. 31:645–651.
Article22. Gläser R, Navid F, Schuller W, Jantschitsch C, Harder J, Schröder JM, et al. UV-B radiation induces the expression of antimicrobial peptides in human keratinocytes in vitro and in vivo. J Allergy Clin Immunol. 2009. 123:1117–1123.
Article23. Zhao C, Wang I, Lehrer RI. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996. 396:319–322.
Article24. Cowland JB, Johnsen AH, Borregaard N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995. 368:173–176.
Article25. Larrick JW, Hirata M, Zhong J, Wright SC. Anti-microbial activity of human CAP18 peptides. Immunotechnology. 1995. 1:65–72.
Article26. Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem. 1996. 238:325–332.
Article27. López-García B, Lee PH, Yamasaki K, Gallo RL. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol. 2005. 125:108–115.
Article28. López-García B, Lee PH, Gallo RL. Expression and potential function of cathelicidin antimicrobial peptides in dermatophytosis and tinea versicolor. J Antimicrob Chemother. 2006. 57:877–882.
Article29. Tsai PW, Yang CY, Chang HT, Lan CY. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PLoS One. 2011. 6:e17755.
Article30. Braff MH, Hawkins MA, Di Nardo A, Lopez-Garcia B, Howell MD, Wong C, et al. Structure-function relationships among human cathelicidin peptides: dissociation of antimicrobial properties from host immunostimulatory activities. J Immunol. 2005. 174:4271–4278.
Article31. Zheng Y, Niyonsaba F, Ushio H, Nagaoka I, Ikeda S, Okumura K, et al. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br J Dermatol. 2007. 157:1124–1131.
Article32. Yu J, Mookherjee N, Wee K, Bowdish DM, Pistolic J, Li Y, et al. Host defense peptide LL-37, in synergy with inflammatory mediator IL-1beta, augments immune responses by multiple pathways. J Immunol. 2007. 179:7684–7691.
Article33. Koczulla R, von Degenfeld G, Kupatt C, Krötz F, Zahler S, Gloe T, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003. 111:1665–1672.
Article34. Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2008. 122:261–266.
Article35. Oppenheim JJ, Tewary P, de la Rosa G, Yang D. Alarmins initiate host defense. Adv Exp Med Biol. 2007. 601:185–194.
Article36. Tomasinsig L, Pizzirani C, Skerlavaj B, Pellegatti P, Gulinelli S, Tossi A, et al. The human cathelicidin LL-37 modulates the activities of the P2X7 receptor in a structure-dependent manner. J Biol Chem. 2008. 283:30471–30481.
Article37. Di Nardo A, Braff MH, Taylor KR, Na C, Granstein RD, McInturff JE, et al. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J Immunol. 2007. 178:1829–1834.
Article38. Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005. 175:4662–4668.
Article39. Bieber T. Atopic dermatitis. N Engl J Med. 2008. 358:1483–1494.
Article40. Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002. 347:1151–1160.
Article41. Mallbris L, Carlén L, Wei T, Heilborn J, Nilsson MF, Granath F, et al. Injury downregulates the expression of the human cathelicidin protein hCAP18/LL-37 in atopic dermatitis. Exp Dermatol. 2010. 19:442–449. Epub 2009 Jul 23.
Article42. Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006. 24:341–348.
Article43. Jensen JM, Ahrens K, Meingassner J, Scherer A, Bräutigam M, Stütz A, et al. Differential suppression of epidermal antimicrobial protein expression in atopic dermatitis and in EFAD mice by pimecrolimus compared to corticosteroids. Exp Dermatol. 2011. 20:783–788.
Article44. Ballardini N, Johansson C, Lilja G, Lindh M, Linde Y, Scheynius A, et al. Enhanced expression of the antimicrobial peptide LL-37 in lesional skin of adults with atopic eczema. Br J Dermatol. 2009. 161:40–47.
Article45. Goo J, Ji JH, Jeon H, Kim MJ, Jeon SY, Cho MY, et al. Expression of antimicrobial peptides such as LL-37 and hBD-2 in nonlesional skin of atopic individuals. Pediatr Dermatol. 2010. 27:341–348.
Article46. Leung TF, Ching KW, Kong AP, Wong GW, Chan JC, Hon KL. Circulating LL-37 is a biomarker for eczema severity in children. J Eur Acad Dermatol Venereol. 2012. 26:518–522.
Article47. Howell MD, Wollenberg A, Gallo RL, Flaig M, Streib JE, Wong C, et al. Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol. 2006. 117:836–841.
Article48. Hata TR, Kotol P, Boguniewicz M, Taylor P, Paik A, Jackson M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010. 163:659–661.
Article49. Chosidow O, Cribier B. Epidemiology of rosacea: updated data. Ann Dermatol Venereol. 2011. 138:Suppl 2. S124–S128.
Article50. Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007. 13:975–980.
Article51. Murakami M, Lopez-Garcia B, Braff M, Dorschner RA, Gallo RL. Postsecretory processing generates multiple cathelicidins for enhanced topical antimicrobial defense. J Immunol. 2004. 172:3070–3077.
Article52. Meyer-Hoffert U, Schröder JM. Epidermal proteases in the pathogenesis of rosacea. J Investig Dermatol Symp Proc. 2011. 15:16–23.
Article53. Morizane S, Yamasaki K, Kabigting FD, Gallo RL. Kallikrein expression and cathelicidin processing are independently controlled in keratinocytes by calcium, vitamin D(3), and retinoic acid. J Invest Dermatol. 2010. 130:1297–1306.
Article54. Schauber J, Gallo RL. The vitamin D pathway: a new target for control of the skin's immune response? Exp Dermatol. 2008. 17:633–639.
Article55. Peric M, Lehmann B, Vashina G, Dombrowski Y, Koglin S, Meurer M, et al. UV-B-triggered induction of vitamin D3 metabolism differentially affects antimicrobial peptide expression in keratinocytes. J Allergy Clin Immunol. 2010. 125:746–749.
Article56. Park K, Elias PM, Oda Y, Mackenzie D, Mauro T, Holleran WM, et al. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin D receptor-independent pathway. J Biol Chem. 2011. 286:34121–34130.
Article57. Yamasaki K, Kanada K, Macleod DT, Borkowski AW, Morizane S, Nakatsuji T, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011. 131:688–697.
Article58. Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J Immunol. 2008. 181:4279–4286.
Article59. Georgala S, Katoulis AC, Kylafis GD, Koumantaki-Mathioudaki E, Georgala C, Aroni K. Increased density of Demodex folliculorum and evidence of delayed hypersensitivity reaction in subjects with papulopustular rosacea. J Eur Acad Dermatol Venereol. 2001. 15:441–444.
Article60. Hollox EJ, Huffmeier U, Zeeuwen PL, Palla R, Lascorz J, Rodijk-Olthuis D, et al. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet. 2008. 40:23–25.
Article61. Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007. 449:564–569.
Article62. Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Göss C, Anz D, et al. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med. 2011. 3:82ra38.
Article63. Emelianov VU, Bechara FG, Gläser R, Langan EA, Taungjaruwinai WM, Schröder JM, et al. Immunohistological pointers to a possible role for excessive cathelicidin (LL-37) expression by apocrine sweat glands in the pathogenesis of hidradenitis suppurativa/acne inversa. Br J Dermatol. 2011. [Epub ahead of print].
Article64. Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005. 19:1067–1077.
Article65. Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004. 173:2909–2912.
Article66. Peric M, Koglin S, Dombrowski Y, Gross K, Bradac E, Büchau A, et al. Vitamin D analogs differentially control antimicrobial peptide/"alarmin" expression in psoriasis. PLoS One. 2009. 4:e6340.67. Reinholz M, Ruzicka T, Schauber J. Vitamin D and its role in allergic disease. Clin Exp Allergy. 2011. [Epub ahead of print].
Article68. Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, Udall D, et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol. 2008. 122:829–831.
Article69. Hartmann B, Riedel R, Jörss K, Loddenkemper C, Steinmeyer A, Zügel U, et al. Vitamin D receptor activation improves allergen-triggered eczema in mice. J Invest Dermatol. 2012. 132:330–336.
Article70. Vähävihu K, Ala-Houhala M, Peric M, Karisola P, Kautiainen H, Hasan T, et al. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br J Dermatol. 2010. 163:321–328.
Article71. Lazaridou E, Giannopoulou C, Fotiadou C, Vakirlis E, Trigoni A, Ioannides D. The potential role of microorganisms in the development of rosacea. J Dtsch Dermatol Ges. 2011. 9:21–25.
Article72. van Zuuren EJ, Gupta AK, Gover MD, Graber M, Hollis S. Systematic review of rosacea treatments. J Am Acad Dermatol. 2007. 56:107–115.
Article73. Del Rosso JQ, Webster GF, Jackson M, Rendon M, Rich P, Torok H, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007. 56:791–802.
Article74. Graupe K, Cunliffe WJ, Gollnick HP, Zaumseil RP. Efficacy and safety of topical azelaic acid (20 percent cream): an overview of results from European clinical trials and experimental reports. Cutis. 1996. 57:1 Suppl. 20–35.75. Yamasaki K, Gallo R. Azelaic acid gel 15% alters kallikrein 5 and cathelicidin expression in epidermal keratinocytes, critical elements in the pathogenesis of rosacea. J Am Acad Dermatol. 2010. 60:AB1.76. Jansen T, Krug S, Kind P, Plewig G, Messer G. BsmI polymorphism of the vitamin D receptor gene in patients with the fulminant course of rosacea conglobata (rosacea fulminans). J Dermatol. 2004. 31:244–246.
Article77. Segaert S. Vitamin D regulation of cathelicidin in the skin: toward a renaissance of vitamin D in dermatology? J Invest Dermatol. 2008. 128:773–775.
Article78. Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, Facchinetti V, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med. 2009. 206:1983–1994.
Article79. Lebwohl M, Menter A, Koo J, Feldman SR. Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol. 2004. 50:416–430.
Article80. Kim SK, Park S, Lee ES. Toll-like receptors and antimicrobial peptides expressions of psoriasis: correlation with serum vitamin D level. J Korean Med Sci. 2010. 25:1506–1512.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increased Expression of Cathelicidin by Direct Activation of Protease-Activated Receptor 2: Possible Implications on the Pathogenesis of Rosacea
- Vitamin D, the Cutaneous Barrier, Antimicrobial Peptides and Allergies: Is There a Link?
- Up-regulation of Cathelicidin in Chronic Sialadenitis
- Antimicrobial Peptide (LL-37) Expression In Nasal Mucosa
- Acne Inversa: Evaluating Antimicrobial Peptides and Proteins