Ann Dermatol.
2012 Nov;24(4):393-397. 10.5021/ad.2012.24.4.393.
Acne Inversa: Evaluating Antimicrobial Peptides and Proteins
- Affiliations
-
- 1Department of Dermatology, Venerology and Allergology, Ruhr-University Bochum, Bochum, Germany. f.bechara@klinikum-bochum.de
- KMID: 2266032
- DOI: http://doi.org/10.5021/ad.2012.24.4.393
Abstract
- BACKGROUND
Acne inversa is a chronic, suppurative relapsing inflammatory skin disease that primarily affects the axillae, perineum and inframammary regions. Evidence suggests that the innate immune system is involved in the pathogenesis of acne inversa.
OBJECTIVE
To investigate the role of the innate immune system in acne inversa.
METHODS
Skin biopsies were obtained from inflammatory skin lesions (n=17) and from non-lesional skin (intraindividual control, n=17) of patients with acne inversa. Additional skin lesions were taken from patients with chronic venous leg ulcers (interindividual control, n=5). Quantitative real-time reverse transcription-polymerase chain reaction was used to determine the mRNA levels of antimicrobial peptides and proteins (AMPs), including human beta-defensin (hBD)-1, hBD-2 and hBD-3, LL-37 (cathelicidin) and Ribonuclease 7 (RNase 7). mRNA levels were also determined for inflammatory and anti-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-alpha), matrix metalloproteinase-1 (MMP1), interleukin (IL)-1beta, IL-6, IL-8 and IL-10.
RESULTS
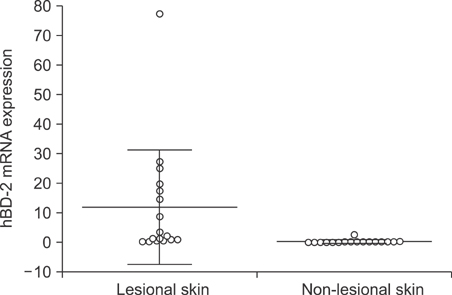
The mRNA levels of hBD-2, LL-37, IL-1beta, IL-6, IL-8, IL-10 and MMP1 were significantly higher in acne inversa lesions compared to non-lesional skin (p<0.05). A significant positive correlation expression was observed between hBD-2 mRNA expression and LL-37 (rho=0.53, p=0.03), and between hBD-2 and RNAse 7 (rho=0.68, p=0.006). When compared to the chronic venous leg ulcer lesions, acne inversa lesions showed a significantly higher expression of RNase 7 mRNA, while IL-1 beta, IL-6, IL-8, TNF-alpha and MMP1 mRNA expression was significantly higher in the chronic venous leg ulcer lesions (p<0.05).
CONCLUSION
The AMP, cytokine milieu and tissue proteases in acne inversa lesions differ significantly from non-lesional skin and chronic venous leg ulcers. The positively correlating up-regulation of AMPs in acne inversa indicates an important role of the innate immune system in the pathogenesis of this disorder.
Keyword
MeSH Terms
-
Acne Vulgaris
Axilla
Biopsy
Cytokines
Hidradenitis Suppurativa
Humans
Immune System
Interleukin-10
Interleukin-1beta
Interleukin-6
Interleukin-8
Interleukins
Leg Ulcer
Matrix Metalloproteinase 1
Peptide Hydrolases
Peptides
Perineum
Proteins
Ribonucleases
RNA, Messenger
Skin
Skin Diseases
Tumor Necrosis Factor-alpha
Up-Regulation
Cytokines
Interleukin-10
Interleukin-1beta
Interleukin-6
Interleukin-8
Interleukins
Matrix Metalloproteinase 1
Peptide Hydrolases
Peptides
Proteins
RNA, Messenger
Ribonucleases
Tumor Necrosis Factor-alpha
Figure
Cited by 1 articles
-
HR-1 Mice: A New Inflammatory Acne Mouse Model
Yong Hyun Jang, Kyou Chae Lee, Seok-Jong Lee, Do Won Kim, Weon Ju Lee
Ann Dermatol. 2015;27(3):257-264. doi: 10.5021/ad.2015.27.3.257.
Reference
-
1. Bechara FG, Hartschuh W. Acne inversa. Hautarzt. 2010. 61:39–46.
Article2. ANderson MJ Jr, Dockerty MB. Perianal hidradenitis suppurativa; a clinical and pathologic study. Dis Colon Rectum. 1958. 1:23–31.3. Kurzen H, Kurokawa I, Jemec GB, Emtestam L, Sellheyer K, Giamarellos-Bourboulis EJ, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008. 17:455–456.
Article4. Schlapbach C, Yawalkar N, Hunger RE. Human beta-defensin-2 and psoriasin are overexpressed in lesions of acne inversa. J Am Acad Dermatol. 2009. 61:58–65.
Article5. Beisswenger C, Kandler K, Hess C, Garn H, Felgentreff K, Wegmann M, et al. Allergic airway inflammation inhibits pulmonary antibacterial host defense. J Immunol. 2006. 177:1833–1837.
Article6. Dann SM, Eckmann L. Innate immune defenses in the intestinal tract. Curr Opin Gastroenterol. 2007. 23:115–120.
Article7. Menendez A, Brett Finlay B. Defensins in the immunology of bacterial infections. Curr Opin Immunol. 2007. 19:385–391.
Article8. de Jongh GJ, Zeeuwen PL, Kucharekova M, Pfundt R, van der Valk PG, Blokx W, et al. High expression levels of keratinocyte antimicrobial proteins in psoriasis compared with atopic dermatitis. J Invest Dermatol. 2005. 125:1163–1173.
Article9. Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001. 25:386–401.
Article10. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001. 25:402–408.
Article11. Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002. 347:1151–1160.
Article12. Gambichler T, Skrygan M, Tigges C, Kobus S, Gläser R, Kreuter A. Significant upregulation of antimicrobial peptides and proteins in lichen sclerosus. Br J Dermatol. 2009. 161:1136–1142.
Article13. Gambichler T, Skrygan M, Tomi NS, Othlinghaus N, Brockmeyer NH, Altmeyer P, et al. Differential mRNA expression of antimicrobial peptides and proteins in atopic dermatitis as compared to psoriasis vulgaris and healthy skin. Int Arch Allergy Immunol. 2008. 147:17–24.
Article14. Kreuter A, Jaouhar M, Skrygan M, Tigges C, Stücker M, Altmeyer P, et al. Expression of antimicrobial peptides in different subtypes of cutaneous lupus erythematosus. J Am Acad Dermatol. 2011. 65:125–133.
Article15. Frohm M, Agerberth B, Ahangari G, Stâhle-Bäckdahl M, Lidén S, Wigzell H, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997. 272:15258–15263.
Article16. Schröder JM, Harder J. Antimicrobial skin peptides and proteins. Cell Mol Life Sci. 2006. 63:469–486.
Article17. Mathews M, Jia HP, Guthmiller JM, Losh G, Graham S, Johnson GK, et al. Production of beta-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect Immun. 1999. 67:2740–2745.
Article18. Kesting MR, Stoeckelhuber M, Hölzle F, Mücke T, Neumann K, Woermann K, et al. Expression of antimicrobial peptides in cutaneous infections after skin surgery. Br J Dermatol. 2010. 163:121–127.
Article19. Guarda G, So A. Regulation of inflammasome activity. Immunology. 2010. 130:329–336.
Article20. Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004. 172:4987–4994.
Article21. Kuwano T, Nakao S, Yamamoto H, Tsuneyoshi M, Yamamoto T, Kuwano M, et al. Cyclooxygenase 2 is a key enzyme for inflammatory cytokine-induced angiogenesis. FASEB J. 2004. 18:300–310.
Article22. Cumberbatch M, Dearman RJ, Griffiths CE, Kimber I. Langerhans cell migration. Clin Exp Dermatol. 2000. 25:413–418.
Article23. Sauter W, Rosenberger A, Beckmann L, Kropp S, Mittelstrass K, Timofeeva M, et al. Matrix metalloproteinase 1 (MMP1) is associated with early-onset lung cancer. Cancer Epidemiol Biomarkers Prev. 2008. 17:1127–1135.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Novel Mutation of the NCSTN Gene Identified in a Chinese Acne Inversa Family
- Antimicrobial Peptides in Innate Immunity against Mycobacteria
- Expression of Antimicrobial Peptides and Proteins in Epidermis Equivalents Exposed to Salt Water and Narrowband Ultraviolet B Radiation
- Acne Conglobata Induced by Anabolic Androgenic Steroids
- Development and Application of Cell-penetrating Peptides