Korean J Physiol Pharmacol.
2018 Nov;22(6):661-670. 10.4196/kjpp.2018.22.6.661.
Fimasartan attenuates renal ischemia-reperfusion injury by modulating inflammation-related apoptosis
- Affiliations
-
- 1Department of Internal Medicine, School of Medicine, Kyungpook National University, Daegu 41944, Korea. ylkim@knu.ac.kr
- KMID: 2430088
- DOI: http://doi.org/10.4196/kjpp.2018.22.6.661
Abstract
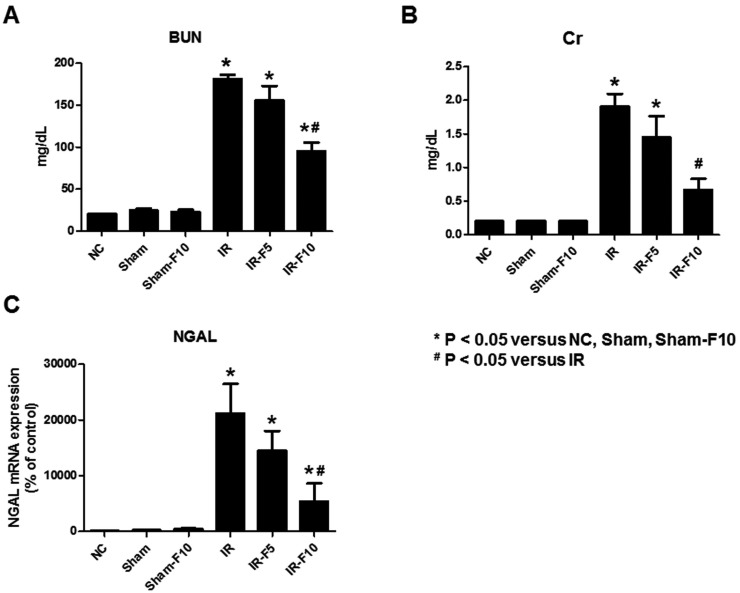
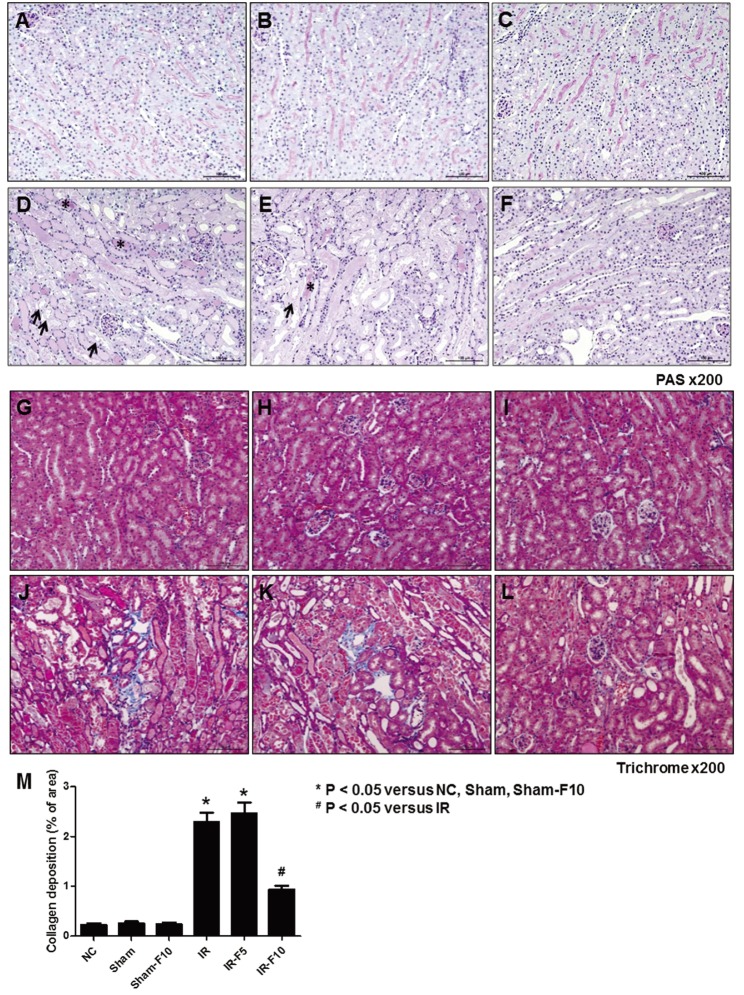
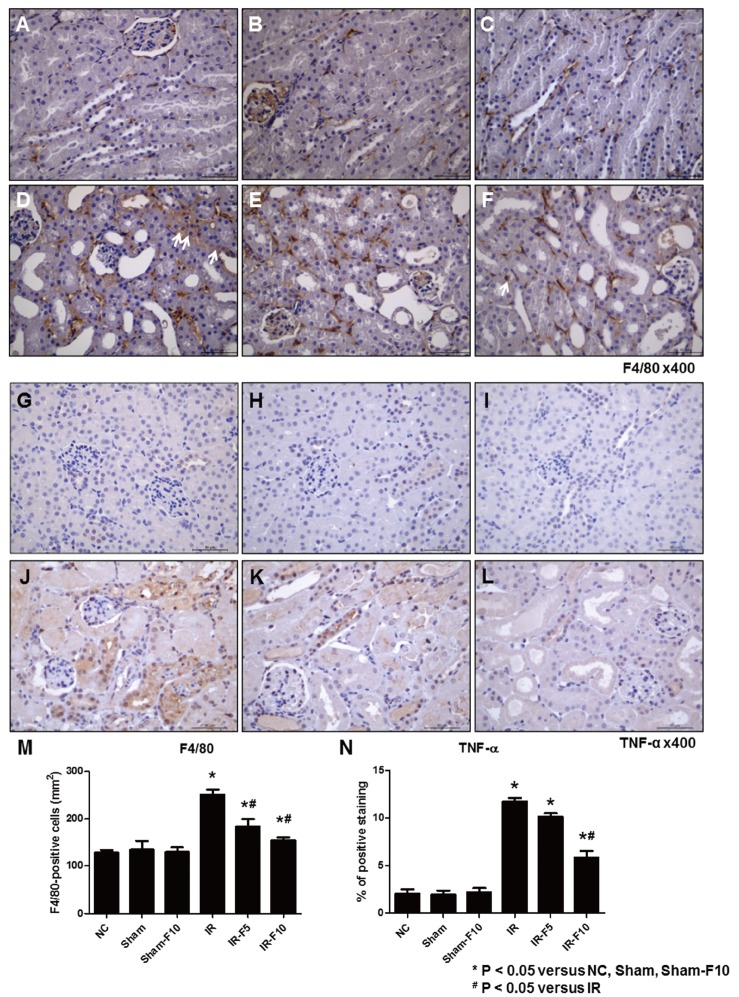
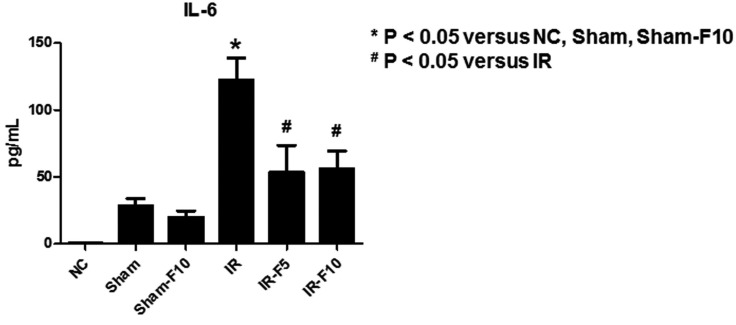
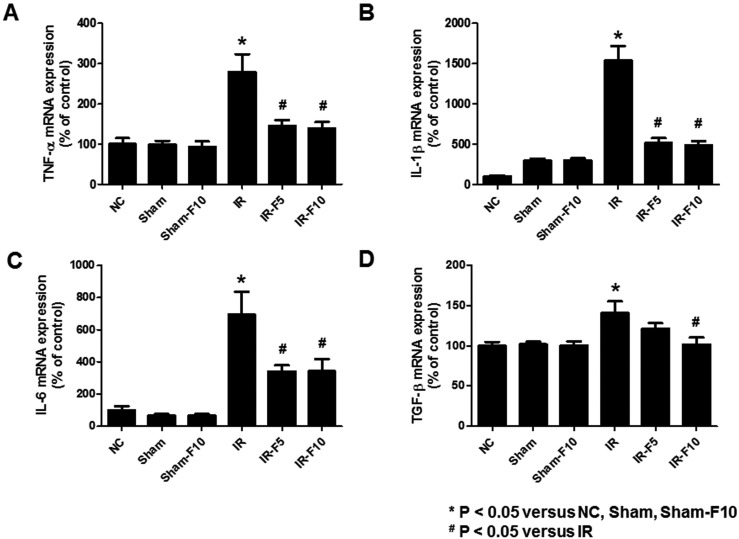
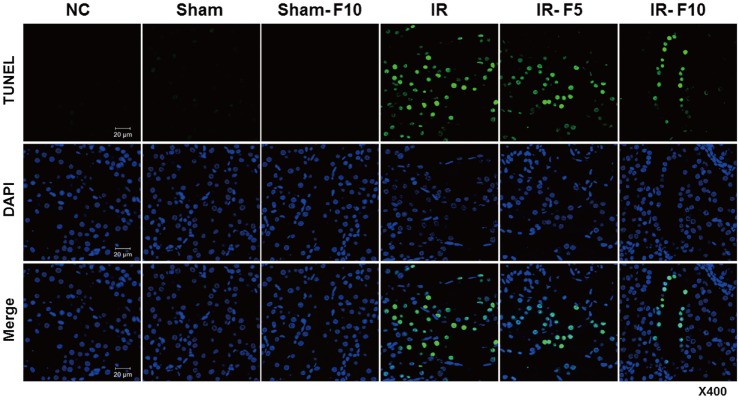
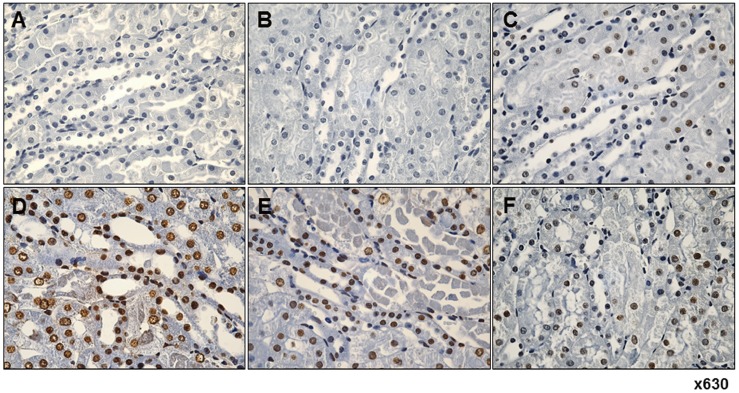
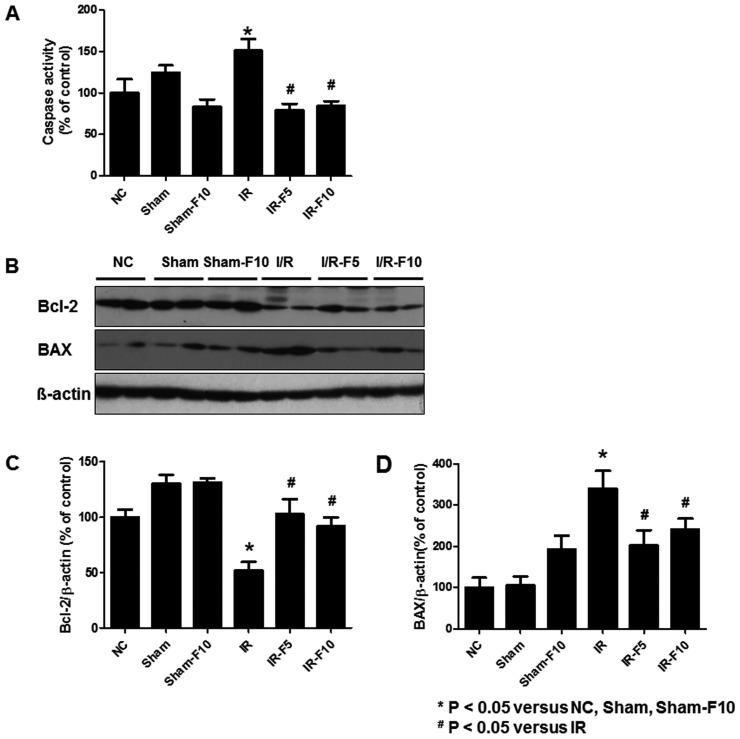
- Fimasartan, a new angiotensin II receptor antagonist, reduces myocyte damage and stabilizes atherosclerotic plaque through its anti-inflammatory effect in animal studies. We investigated the protective effects of pretreatment with fimasartan on ischemia-reperfusion injury (IRI) in a mouse model of ischemic renal damage. C57BL/6 mice were pretreated with or without 5 (IR-F5) or 10 (IR-F10) mg/kg/day fimasartan for 3 days. Renal ischemia was induced by clamping bilateral renal vascular pedicles for 30 min. Histology, pro-inflammatory cytokines, and apoptosis assays were evaluated 24 h after IRI. Compared to the untreated group, blood urea nitrogen and serum creatinine levels were significantly lower in the IR-F10 group. IR-F10 kidneys showed less tubular necrosis and interstitial fibrosis than untreated kidneys. The expression of F4/80, a macrophage infiltration marker, and tumor necrosis factor (TNF)-α, decreased in the IR-F10 group. High-dose fimasartan treatment attenuated the upregulation of TNF-α, interleukin (IL)-1β, and IL-6 in ischemic kidneys. Fewer TUNEL positive cells were observed in IR-F10 compared to control mice. Fimasartan caused a significant decrease in caspase-3 activity and the level of Bax, and increased the Bcl-2 level. Fimasartan preserved renal function and tubular architecture from IRI in a mouse ischemic renal injury model. Fimasartan also attenuated upregulation of inflammatory cytokines and decreased apoptosis of renal tubular cells. Our results suggest that fimasartan inhibited the process of tubular injury by preventing apoptosis induced by the inflammatory pathway.
MeSH Terms
-
Animals
Apoptosis*
Blood Urea Nitrogen
Caspase 3
Constriction
Creatinine
Cytokines
Fibrosis
In Situ Nick-End Labeling
Interleukin-6
Interleukins
Ischemia
Kidney
Macrophages
Mice
Muscle Cells
Necrosis
Plaque, Atherosclerotic
Receptors, Angiotensin
Reperfusion Injury*
Tumor Necrosis Factor-alpha
Up-Regulation
Caspase 3
Creatinine
Cytokines
Interleukin-6
Interleukins
Receptors, Angiotensin
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Weight SC, Bell PR, Nicholson ML. Renal ischaemia-reperfusion injury. Br J Surg. 1996; 83:162–170. PMID: 8689154.
Article2. Edelstein CL, Ling H, Schrier RW. The nature of renal cell injury. Kidney Int. 1997; 51:1341–1351. PMID: 9150442.
Article3. Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003; 14:2199–2210. PMID: 12874476.
Article4. Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004; 66:480–485. PMID: 15253693.
Article5. Rah DK, Han DW, Baek HS, Hyon SH, Park BY, Park JC. Protection of rabbit kidney from ischemia/reperfusion injury by green tea polyphenol pretreatment. Arch Pharm Res. 2007; 30:1447–1454. PMID: 18087814.
Article6. Cau J, Favreau F, Zhang K, Febrer G, de la Motte GR, Ricco JB, Goujon JM, Hauet T. FR167653 improves renal recovery and decreases inflammation and fibrosis after renal ischemia reperfusion injury. J Vasc Surg. 2009; 49:728–740. PMID: 19268775.
Article7. Korkmaz A, Kolankaya D. The protective effects of ascorbic acid against renal ischemia-reperfusion injury in male rats. Ren Fail. 2009; 31:36–43. PMID: 19142808.
Article8. Kontogiannis J, Burns KD. Role of AT1 angiotensin II receptors in renal ischemic injury. Am J Physiol. 1998; 274:F79–F90. PMID: 9458826.9. Hammad FT, Wheatley AM, Davis G. Role of endothelin ET(A) receptor antagonism in the post-transplant renal response to angiotensin II in the rat. Exp Physiol. 2001; 86:365–372. PMID: 11429654.
Article10. Harrison-Bernard LM, Navar LG, Ho MM, Vinson GP, el-Dahr SS. Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol. 1997; 273:F170–F177. PMID: 9249605.
Article11. Ruiz-Ortega M, Rupérez M, Esteban V, Rodríguez-Vita J, Sánchez-López E, Carvajal G, Egido J. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant. 2006; 21:16–20. PMID: 16280370.
Article12. Habibey R, Ajami M, Hesami A, Pazoki-Toroudi H. The mechanism of preventive effect of captopril on renal ischemia reperfusion injury is independent of ATP dependent potassium channels. Iran Biomed J. 2008; 12:241–245. PMID: 19079539.13. Molinas SM, Cortés-González C, González-Bobadilla Y, Monasterolo LA, Cruz C, Elías MM, Bobadilla NA, Trumper L. Effects of losartan pretreatment in an experimental model of ischemic acute kidney injury. Nephron Exp Nephrol. 2009; 112:e10–e19. PMID: 19342869.
Article14. Fouad AA, Qureshi HA, Al-Sultan AI, Yacoubi MT, Al-Melhim WN. Nephroprotective effect of telmisartan in rats with ischemia/reperfusion renal injury. Pharmacology. 2010; 85:158–167. PMID: 20150754.
Article15. Fimasartan. Am J Cardiovasc Drugs. 2011; 11:249–252. PMID: 21740078.16. Kim TW, Yoo BW, Lee JK, Kim JH, Lee KT, Chi YH, Lee JY. Synthesis and antihypertensive activity of pyrimidin-4(3H)-one derivatives as losartan analogue for new angiotensin II receptor type 1 (AT1) antagonists. Bioorg Med Chem Lett. 2012; 22:1649–1654. PMID: 22264484.
Article17. Shin BS, Kim TH, Paik SH, Chi YH, Lee JH, Tan HK, Choi Y, Kim M, Yoo SD. Simultaneous determination of fimasartan, a novel antihypertensive agent, and its active metabolite in rat plasma by liquid chromatography-tandem mass spectrometry. Biomed Chromatogr. 2011; 25:1208–1214. PMID: 21268050.
Article18. Shin KH, Kim TE, Kim SE, Lee MG, Song IS, Yoon SH, Cho JY, Jang IJ, Shin SG, Yu KS. The effect of the newly developed angiotensin receptor II antagonist fimasartan on the pharmacokinetics of atorvastatin in relation to OATP1B1 in healthy male volunteers. J Cardiovasc Pharmacol. 2011; 58:492–499. PMID: 21765368.
Article19. Yi S, Kim JW, Kim TE, Kim J, Jun YK, Choi J, Yoon SH, Cho JY, Song SH, Shin SG, Jang IJ, Yu KS. Effect of multiple doses of fimasartan, an angiotensin II receptor antagonist, on the steady-state pharmacokinetics of digoxin in healthy volunteers. Int J Clin Pharmacol Ther. 2011; 49:321–327. PMID: 21543035.
Article20. Yi S, Kim TE, Yoon SH, Cho JY, Shin SG, Jang IJ, Yu KS. Pharmacokinetic interaction of fimasartan, a new angiotensin II receptor antagonist, with amlodipine in healthy volunteers. J Cardiovasc Pharmacol. 2011; 57:682–689. PMID: 21394036.
Article21. Han J, Park SJ, Thu VT, Lee SR, Long le T, Kim HK, Kim N, Park SW, Jeon ES, Kim EJ, Yoon CH, Cho GY, Choi DJ. Effects of the novel angiotensin II receptor type I antagonist, fimasartan on myocardial ischemia/reperfusion injury. Int J Cardiol. 2013; 168:2851–2859. PMID: 23642815.
Article22. Lee JY, Lee CW, Kim WJ, Ahn JM, Park DW, Kang SJ, Lee SW, Kim YH, Son WC, Jung S, Park SW, Park SJ. Antiatherosclerotic effects of the novel angiotensin receptor antagonist Fimasartan on plaque progression and stability in a rabbit model: a double-blind placebo-controlled trial. J Cardiovasc Pharmacol. 2013; 62:229–236. PMID: 23615162.23. Chang SA, Lim BK, Lee YJ, Hong MK, Choi JO, Jeon ES. A novel angiotensin type I receptor antagonist, fimasartan, prevents doxorubicin-induced cardiotoxicity in rats. J Korean Med Sci. 2015; 30:559–568. PMID: 25931786.
Article24. Kim S, Kim SJ, Yoon HE, Chung S, Choi BS, Park CW, Shin SJ. Fimasartan, a novel angiotensin-receptor blocker, protects against renal inflammation and fibrosis in mice with unilateral ureteral obstruction: the possible role of Nrf2. Int J Med Sci. 2015; 12:891–904. PMID: 26640409.
Article25. Donnahoo KK, Meng X, Ayala A, Cain MP, Harken AH, Meldrum DR. Early kidney TNF-alpha expression mediates neutrophil infiltration and injury after renal ischemia-reperfusion. Am J Physiol. 1999; 277:R922–R929. PMID: 10484513.26. Donnahoo KK, Shames BD, Harken AH, Meldrum DR. Review article: the role of tumor necrosis factor in renal ischemia-reperfusion injury. J Urol. 1999; 162:196–203. PMID: 10379787.
Article27. Rabb H, O’Meara YM, Maderna P, Coleman P, Brady HR. Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int. 1997; 51:1463–1468. PMID: 9150459.
Article28. Glynne PA, Picot J, Evans TJ. Coexpressed nitric oxide synthase and apical beta(1) integrins influence tubule cell adhesion after cytokine-induced injury. J Am Soc Nephrol. 2001; 12:2370–2383. PMID: 11675413.29. Patel NS, Chatterjee PK, Di Paola R, Mazzon E, Britti D, De Sarro A, Cuzzocrea S, Thiemermann C. Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/ reperfusion. J Pharmacol Exp Ther. 2005; 312:1170–1178. PMID: 15572648.30. Seujange Y, Eiam-Ong S, Tirawatnapong T, Eiam-Ong S. Role of angiotensin II on dihydrofolate reductase, GTP-cyclohydrolase 1 and nitric oxide synthase expressions in renal ischemia-reperfusion. Am J Nephrol. 2008; 28:692–700. PMID: 18408363.
Article31. Park JK, Mervaala EM, Muller DN, Menne J, Fiebeler A, Luft FC, Haller H. Rosuvastatin protects against angiotensin II-induced renal injury in a dose-dependent fashion. J Hypertens. 2009; 27:599–605. PMID: 19262227.
Article32. Basnakian AG, Kaushal GP, Shah SV. Apoptotic pathways of oxidative damage to renal tubular epithelial cells. Antioxid Redox Signal. 2002; 4:915–924. PMID: 12573140.
Article33. Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int. 2011; 80:29–40. PMID: 21562469.
Article34. Saikumar P, Venkatachalam MA. Role of apoptosis in hypoxic/ischemic damage in the kidney. Semin Nephrol. 2003; 23:511–521. PMID: 14631559.
Article35. Kaushal GP, Basnakian AG, Shah SV. Apoptotic pathways in ischemic acute renal failure. Kidney Int. 2004; 66:500–506. PMID: 15253697.
Article36. Faubel S, Ljubanovic D, Reznikov L, Somerset H, Dinarello CA, Edelstein CL. Caspase-1-deficient mice are protected against cisplatin-induced apoptosis and acute tubular necrosis. Kidney Int. 2004; 66:2202–2213. PMID: 15569309.
Article37. Chien CT, Chang TC, Tsai CY, Shyue SK, Lai MK. Adenovirus-mediated bcl-2 gene transfer inhibits renal ischemia/reperfusion induced tubular oxidative stress and apoptosis. Am J Transplant. 2005; 5:1194–1203. PMID: 15888023.
Article38. Xue F, Isaka Y, Takahara T, Imamura R, Suzuki C, Ichimaru N, Michieli P, Takahara S. HGF-MSP chimera protects kidneys from ischemia-reperfusion injury. Biochem Biophys Res Commun. 2007; 363:451–456. PMID: 17888399.
Article39. Havasi A, Li Z, Wang Z, Martin JL, Botla V, Ruchalski K, Schwartz JH, Borkan SC. Hsp27 inhibits Bax activation and apoptosis via a phosphatidylinositol 3-kinase-dependent mechanism. J Biol Chem. 2008; 283:12305–12313. PMID: 18299320.
Article40. Deng A, Miracle CM, Suarez JM, Lortie M, Satriano J, Thomson SC, Munger KA, Blantz RC. Oxygen consumption in the kidney: effects of nitric oxide synthase isoforms and angiotensin II. Kidney Int. 2005; 68:723–730. PMID: 16014049.
Article41. Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008; 9:47–59. PMID: 18097445.
Article42. Nowak G, Schnellmann RG. Autocrine production and TGF-beta 1-mediated effects on metabolism and viability in renal cells. Am J Physiol. 1996; 271:F689–F697. PMID: 8853432.
Article43. Sanderson N, Factor V, Nagy P, Kopp J, Kondaiah P, Wakefield L, Roberts AB, Sporn MB, Thorgeirsson SS. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci U S A. 1995; 92:2572–2576. PMID: 7708687.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mechanisms and therapeutic targets of ischemic acute kidney injury
- Quantitative evaluation of renal parenchymal perfusion using contrast-enhanced ultrasonography in renal ischemia-reperfusion injury in dogs
- Curcumin attenuates renal ischemia reperfusion injury via JNK pathway with the involvement of p300/CBP-mediated histone acetylation
- PGC1-alpha plays a role in hypothermic renal protection of renal fibrosis after acute kidney injury
- Ethyl Pyruvate Ameliorates Renal Ischemia- reperfusion Injury