Korean J Physiol Pharmacol.
2021 Sep;25(5):413-423. 10.4196/kjpp.2021.25.5.413.
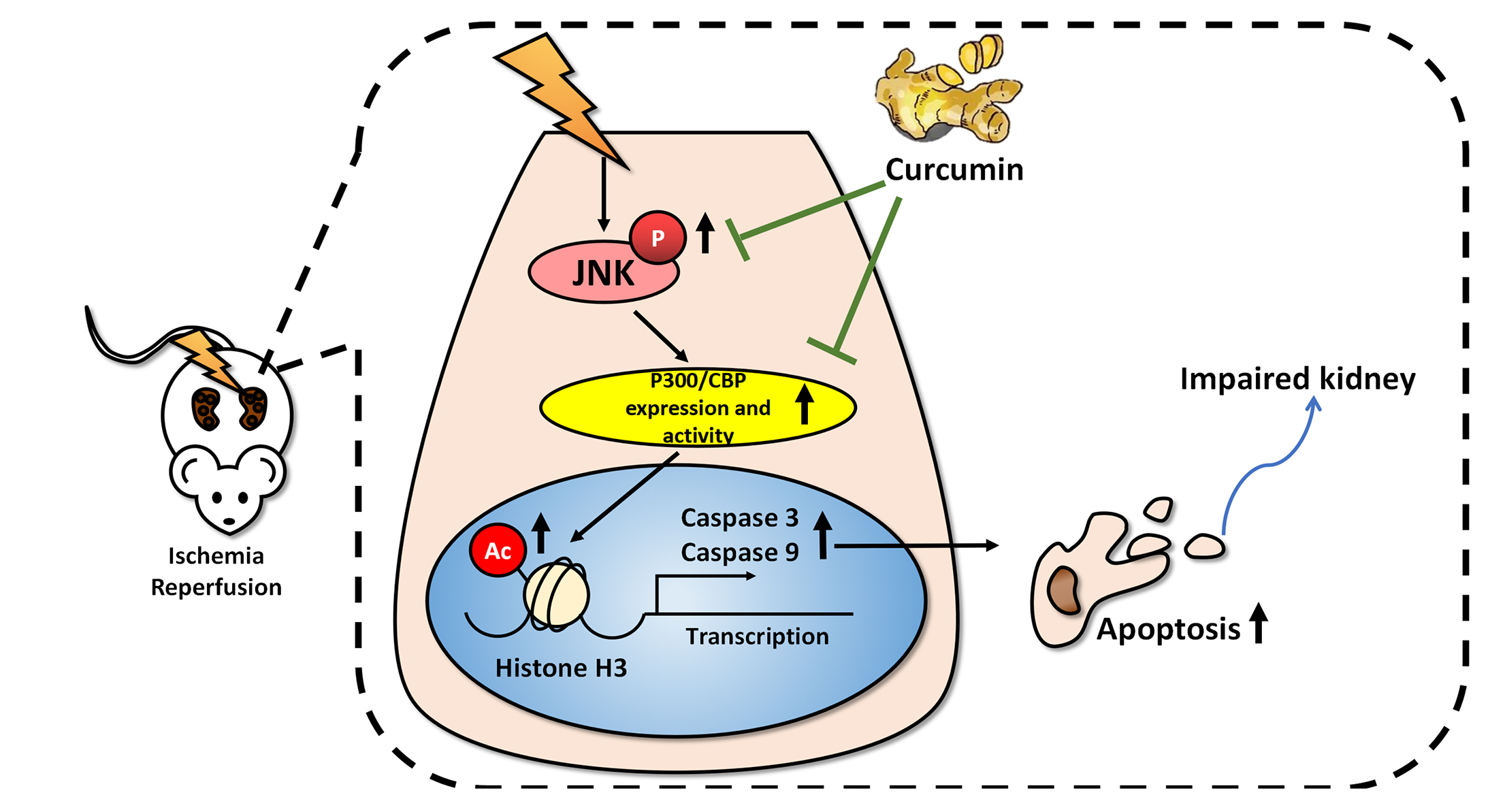
Curcumin attenuates renal ischemia reperfusion injury via JNK pathway with the involvement of p300/CBP-mediated histone acetylation
- Affiliations
-
- 1Department of Anesthesiology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou 510080, P.R. China.
- 2Department of Organ Transplantation, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou 510080, P.R. China.
- 3Department of Organ Transplantation, Zhujiang Hospital of Southern Medical University, Guangzhou 510000, P.R. China.
- KMID: 2519404
- DOI: http://doi.org/10.4196/kjpp.2021.25.5.413
Abstract
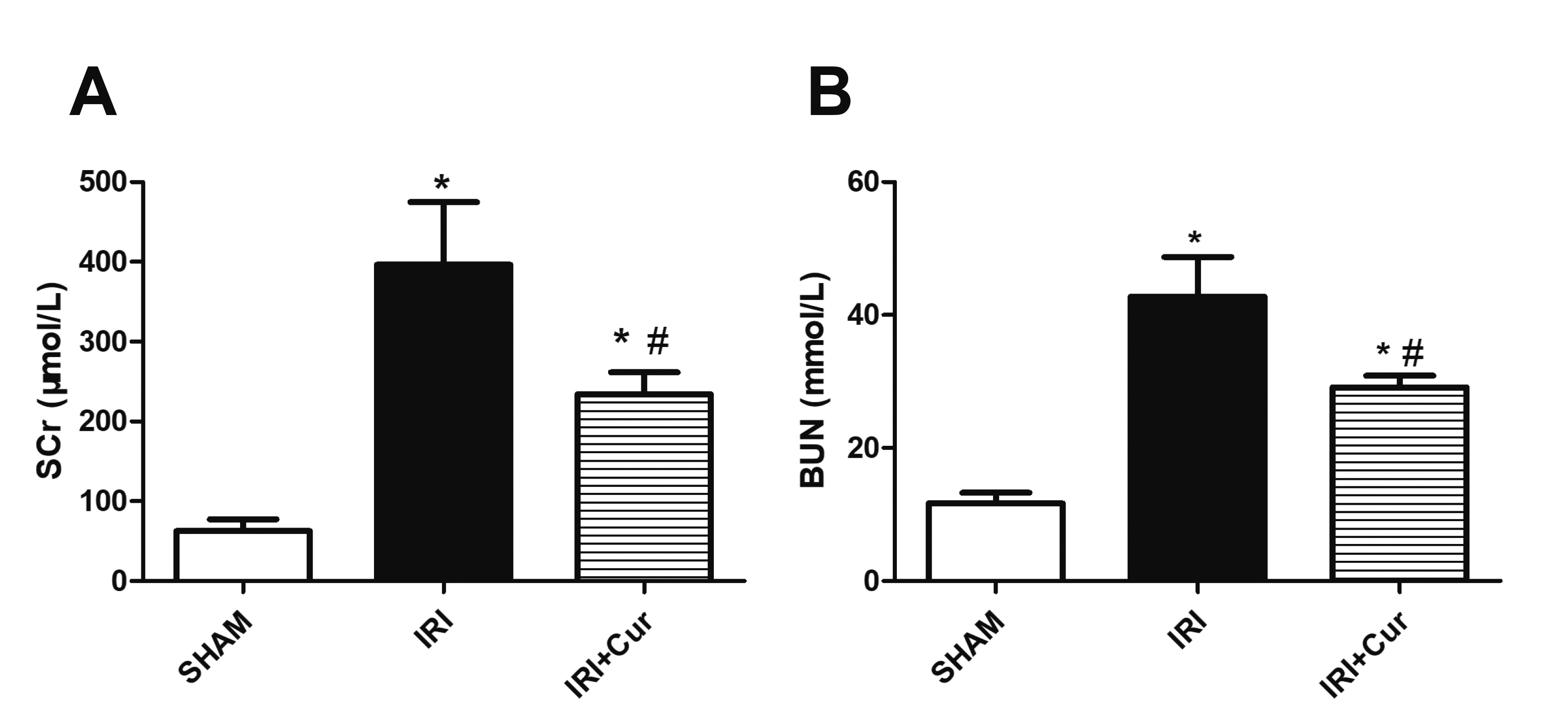
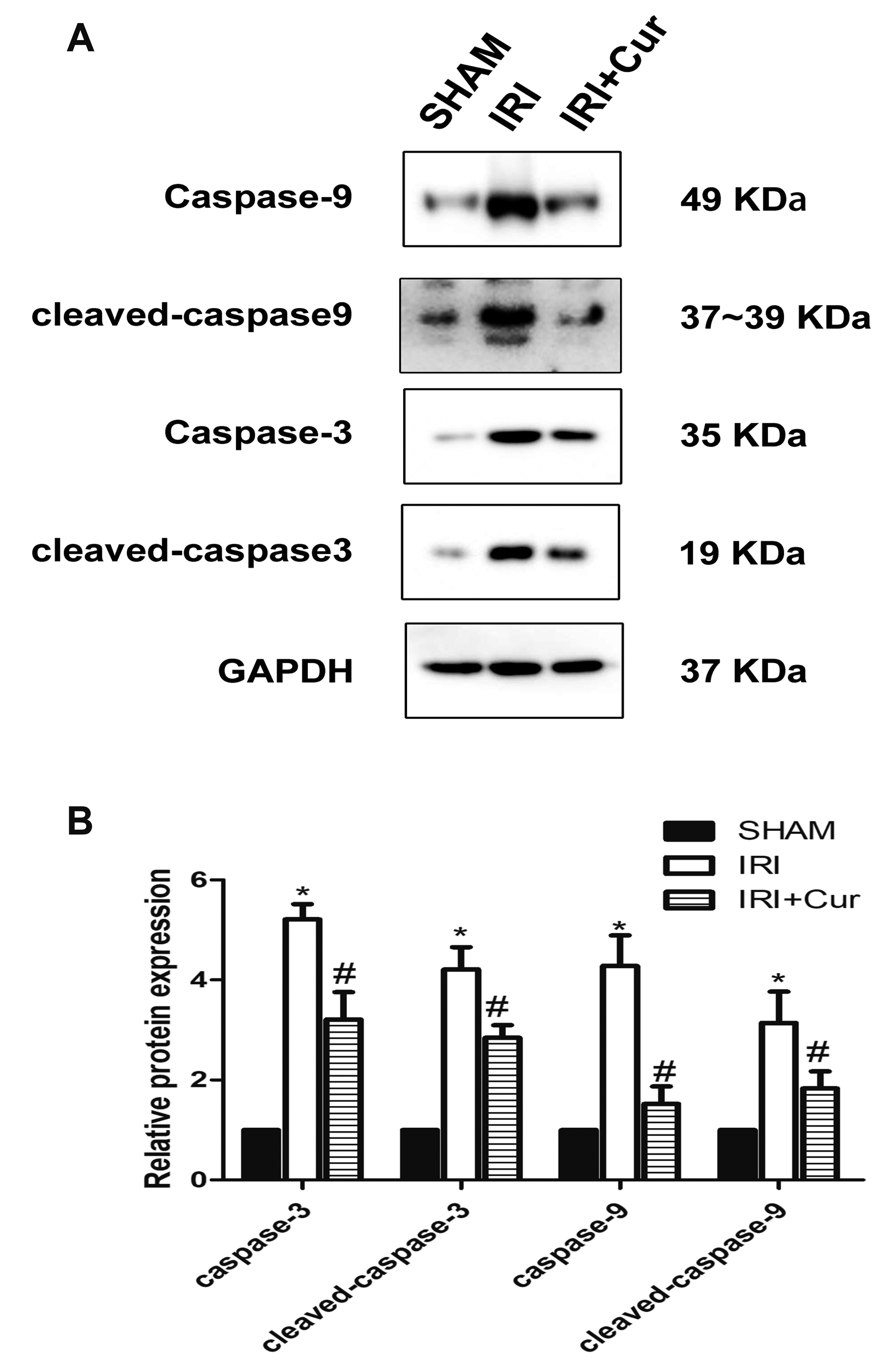
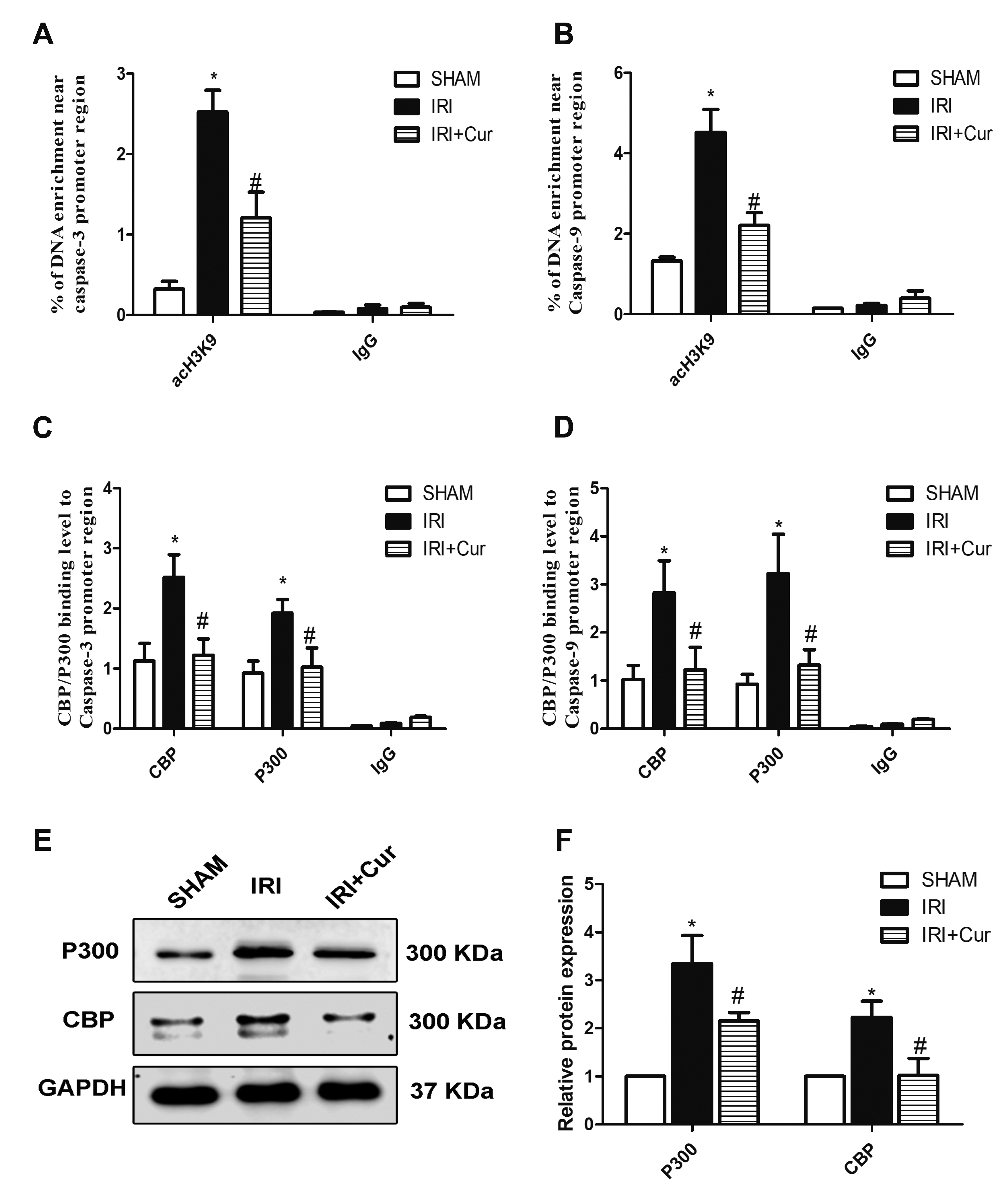
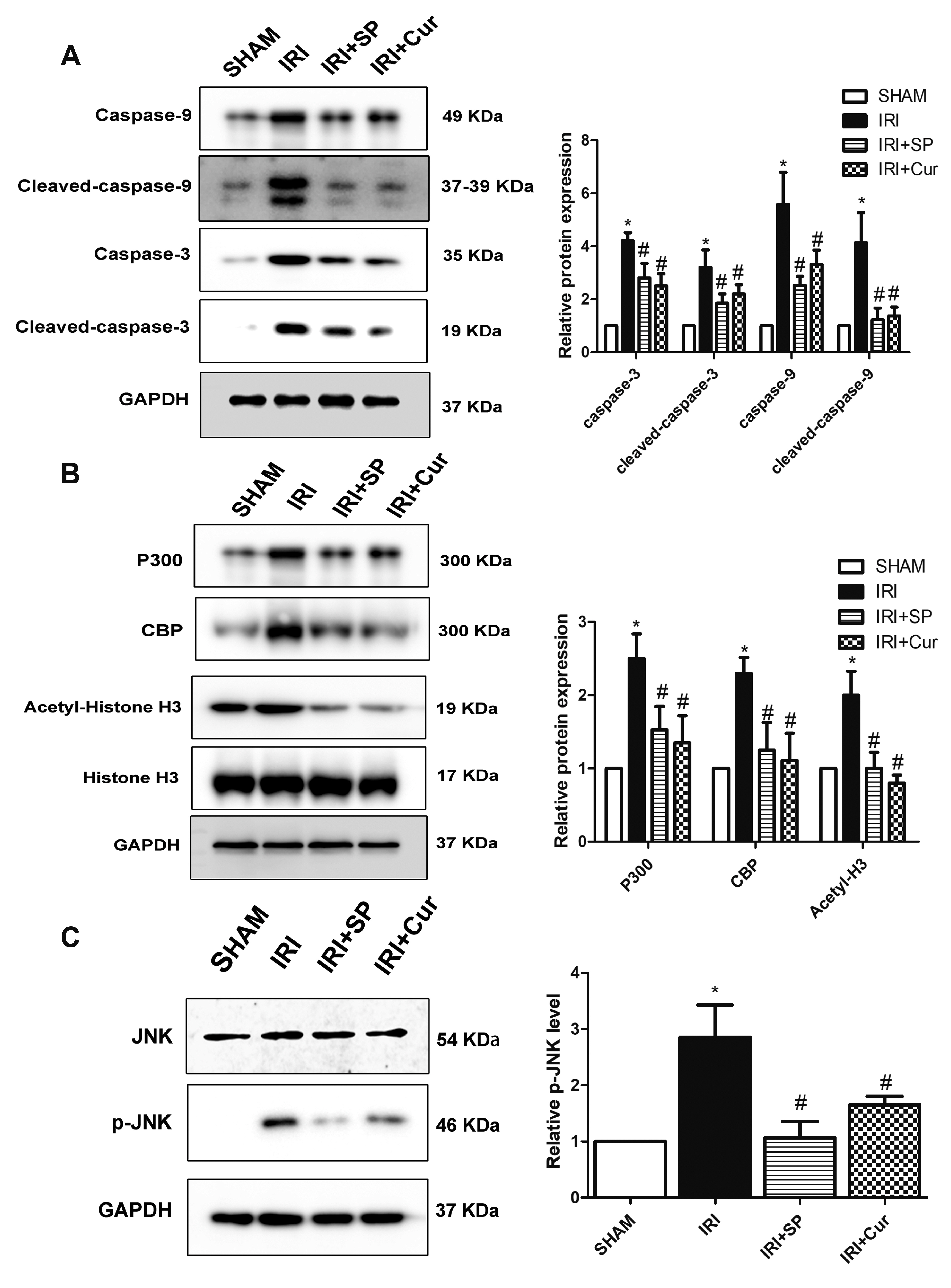
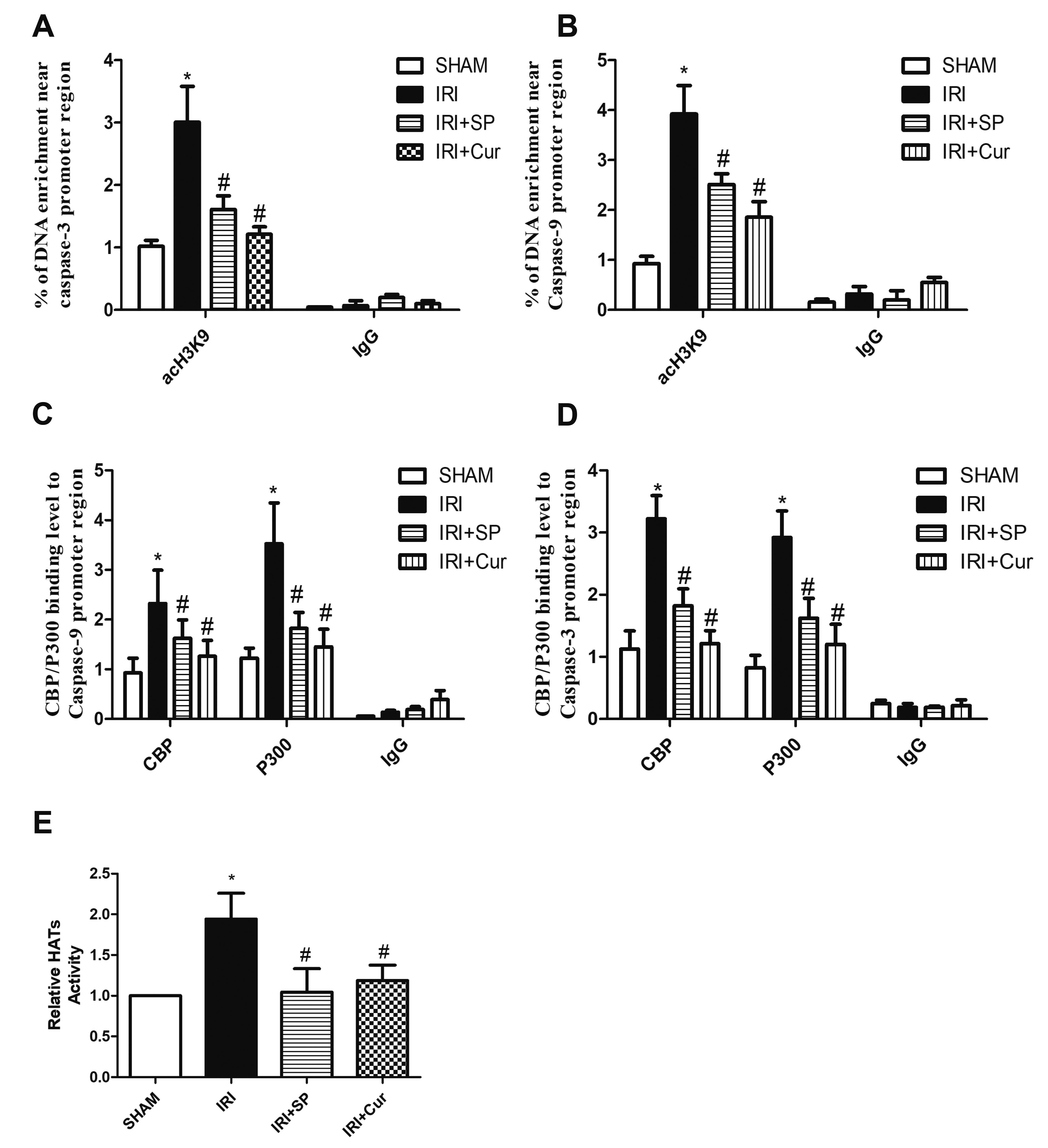
- Apoptosis is proved responsible for renal damage during ischemia/reperfusion. The regulation for renal apoptosis induced by ischemia/reperfusion injury (IRI) has still been unclearly characterized to date. In the present study, we investigated the regulation of histone acetylation on IRI-induced renal apoptosis and the molecular mechanisms in rats with the application of curcumin possessing a variety of biological activities involving inhibition of apoptosis. Sprague–Dawley rats were randomized into four experimental groups (SHAM, IRI, curcumin, SP600125). Results showed that curcumin significantly decreased renal apoptosis and caspase-3/-9 expression and enhanced renal function in IRI rats. Treatment with curcumin in IRI rats also led to the decrease in expression of p300/cyclic AMP response element-binding protein (CBP) and activity of histone acetyltransferases (HATs). Reduced histone H3 lysine 9 (H3K9) acetylation was found near the promoter region of caspase-3/-9 after curcumin treatment. In a similar way, SP600125, an inhibitor of c-Jun N-terminal kinase (JNK), also attenuated renal apoptosis and enhanced renal function in IRI rats. In addition, SP600125 suppressed the binding level of p300/CBP and H3K9 acetylation near the promoter region of caspase-3/-9, and curcumin could inhibit JNK phosphorylation like SP600125. These results indicate that curcumin could attenuate renal IRI via JNK/p300/CBP-mediated anti-apoptosis signaling.
Keyword
Figure
Reference
-
1. Saat TC, van den Akker EK, IJzermans JN, Dor FJ, de Bruin RW. 2016; Improving the outcome of kidney transplantation by ameliorating renal ischemia reperfusion injury: lost in translation? J Transl Med. 14:20. DOI: 10.1186/s12967-016-0767-2. PMID: 26791565. PMCID: PMC4721068.
Article2. Ditonno P, Impedovo SV, Palazzo S, Bettocchi C, Gesualdo L, Grandaliano G, Selvaggi FP, Battaglia M. 2013; Effects of ischemia-reperfusion injury in kidney transplantation: risk factors and early and long-term outcomes in a single center. Transplant Proc. 45:2641–2644. DOI: 10.1016/j.transproceed.2013.07.025. PMID: 24034012.
Article3. Rewa O, Bagshaw SM. 2014; Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 10:193–207. DOI: 10.1038/nrneph.2013.282. PMID: 24445744.
Article4. Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, Goldstein SL, Cerdá J, Chawla LS. 2018; Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 14:607–625. DOI: 10.1038/s41581-018-0052-0. PMID: 30135570.
Article5. Kelly KJ. 2003; Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. 14:1549–1558. DOI: 10.1097/01.ASN.0000064946.94590.46. PMID: 12761255.
Article6. Awad AS, El-Sharif AA. 2011; Curcumin immune-mediated and anti-apoptotic mechanisms protect against renal ischemia/reperfusion and distant organ induced injuries. Int Immunopharmacol. 11:992–996. DOI: 10.1016/j.intimp.2011.02.015. PMID: 21354353.
Article7. Bonegio R, Lieberthal W. 2002; Role of apoptosis in the pathogenesis of acute renal failure. Curr Opin Nephrol Hypertens. 11:301–308. DOI: 10.1097/00041552-200205000-00006. PMID: 11981260.
Article8. Basile DP, Anderson MD, Sutton TA. 2012; Pathophysiology of acute kidney injury. Compr Physiol. 2:1303–1353. DOI: 10.1002/cphy.c110041. PMID: 23798302. PMCID: PMC3919808.
Article9. Zhao Y, Ding C, Xue W, Ding X, Zheng J, Gao Y, Xia X, Li S, Liu J, Han F, Zhu F, Tian P. 2017; Genome-wide DNA methylation analysis in renal ischemia reperfusion injury. Gene. 610:32–43. DOI: 10.1016/j.gene.2017.02.005. PMID: 28189760.
Article10. Fontecha-Barriuso M, Martin-Sanchez D, Ruiz-Andres O, Poveda J, Sanchez-Niño MD, Valiño-Rivas L, Ruiz-Ortega M, Ortiz A, Sanz AB. 2018; Targeting epigenetic DNA and histone modifications to treat kidney disease. Nephrol Dial Transplant. 33:1875–1886. DOI: 10.1093/ndt/gfy009. PMID: 29534238.
Article11. Guo C, Dong G, Liang X, Dong Z. 2019; Epigenetic regulation in AKI and kidney repair: mechanisms and therapeutic implications. Nat Rev Nephrol. 15:220–239. DOI: 10.1038/s41581-018-0103-6. PMID: 30651611. PMCID: PMC7866490.
Article12. Tessarz P, Kouzarides T. 2014; Histone core modifications regulating nucleosome structure and dynamics. Nat Rev Mol Cell Biol. 15:703–708. DOI: 10.1038/nrm3890. PMID: 25315270.
Article13. Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL. 2006; Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 311:844–847. DOI: 10.1126/science.1124000. PMID: 16469925.
Article14. Tang J, Zhuang S. 2019; Histone acetylation and DNA methylation in ischemia/reperfusion injury. Clin Sci (Lond). 133:597–609. DOI: 10.1042/CS20180465. PMID: 30804072. PMCID: PMC7470454.
Article15. Bomsztyk K, Denisenko O. 2013; Epigenetic alterations in acute kidney injury. Semin Nephrol. 33:327–340. DOI: 10.1016/j.semnephrol.2013.05.005. PMID: 24011575. PMCID: PMC3768006.
Article16. Audia JE, Campbell RM. 2016; Histone modifications and cancer. Cold Spring Harb Perspect Biol. 8:a019521. DOI: 10.1101/cshperspect.a019521. PMID: 27037415. PMCID: PMC4817802.
Article17. Bao L, Diao H, Dong N, Su X, Wang B, Mo Q, Yu H, Wang X, Chen C. 2016; Histone deacetylase inhibitor induces cell apoptosis and cycle arrest in lung cancer cells via mitochondrial injury and p53 up-acetylation. Cell Biol Toxicol. 32:469–482. DOI: 10.1007/s10565-016-9347-8. PMID: 27423454. PMCID: PMC5099365.
Article18. Li D, Zeng Z. 2019; Epigenetic regulation of histone H3 in the process of hepatocellular tumorigenesis. Biosci Rep. 39:BSR20191815. DOI: 10.1042/BSR20191815. PMID: 31320544. PMCID: PMC6680372.
Article19. Ammon HP, Wahl MA. 1991; Pharmacology of Curcuma longa. Planta Med. 57:1–7. DOI: 10.1055/s-2006-960004. PMID: 2062949.20. Marcu MG, Jung YJ, Lee S, Chung EJ, Lee MJ, Trepel J, Neckers L. 2006; Curcumin is an inhibitor of p300 histone acetylatransferase. Med Chem. 2:169–174. DOI: 10.2174/157340606776056133. PMID: 16787365.21. Wang X, Muhammad I, Sun X, Han M, Hamid S, Zhang X. 2018; Protective role of curcumin in ameliorating AFB1-induced apoptosis via mitochondrial pathway in liver cells. Mol Biol Rep. 45:881–891. DOI: 10.1007/s11033-018-4234-4. PMID: 29974318.
Article22. Yu W, Zha W, Ke Z, Min Q, Li C, Sun H, Liu C. 2016; Curcumin protects neonatal rat cardiomyocytes against high glucose-induced apoptosis via PI3K/Akt signalling pathway. J Diabetes Res. 2016:4158591. DOI: 10.1155/2016/4158591. PMID: 26989696. PMCID: PMC4771910.
Article23. Kunduzova OR, Bianchi P, Pizzinat N, Escourrou G, Seguelas MH, Parini A, Cambon C. 2002; Regulation of JNK/ERK activation, cell apoptosis, and tissue regeneration by monoamine oxidases after renal ischemia-reperfusion. FASEB J. 16:1129–1131. DOI: 10.1096/fj.01-1008fje. PMID: 12039844.
Article24. Wu J, Zhang X, Nauta HJ, Lin Q, Li J, Fang L. 2008; JNK1 regulates histone acetylation in trigeminal neurons following chemical stimulation. Biochem Biophys Res Commun. 376:781–786. DOI: 10.1016/j.bbrc.2008.09.073. PMID: 18822271. PMCID: PMC2702224.
Article25. Bayrak O, Uz E, Bayrak R, Turgut F, Atmaca AF, Sahin S, Yildirim ME, Kaya A, Cimentepe E, Akcay A. 2008; Curcumin protects against ischemia/reperfusion injury in rat kidneys. World J Urol. 26:285–291. DOI: 10.1007/s00345-008-0253-4. PMID: 18373094.
Article26. Wu J, Pan X, Fu H, Zheng Y, Dai Y, Yin Y, Chen Q, Hao Q, Bao D, Hou D. 2017; Effect of curcumin on glycerol-induced acute kidney injury in rats. Sci Rep. 7:10114. DOI: 10.1038/s41598-017-10693-4. PMID: 28860665. PMCID: PMC5579036.
Article27. Xu YF, Liu M, Peng B, Che JP, Zhang HM, Yan Y, Wang GC, Wu YC, Zheng JH. 2011; Protective effects of SP600125 on renal ischemia-reperfusion injury in rats. J Surg Res. 169:e77–e84. DOI: 10.1016/j.jss.2011.02.021. PMID: 21492872.
Article28. Zhao W, Wu X, Wang Z, Pan B, Liu L, Liu L, Huang X, Tian J. 2020; Epigenetic regulation of phosphodiesterase 4d in restrictive cardiomyopathy mice with cTnI mutations. Sci China Life Sci. 63:563–570. DOI: 10.1007/s11427-018-9463-9. PMID: 30900165.
Article29. Zhou W, Jiang D, Tian J, Liu L, Lu T, Huang X, Sun H. 2018; Acetylation of H3K4, H3K9, and H3K27 mediated by p300 regulates the expression of GATA4 in cardiocytes. Genes Dis. 6:318–325. DOI: 10.1016/j.gendis.2018.10.002. PMID: 32042871. PMCID: PMC6997570.
Article30. Gupta SC, Patchva S, Aggarwal BB. 2013; Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 15:195–218. DOI: 10.1208/s12248-012-9432-8. PMID: 23143785. PMCID: PMC3535097.
Article31. Hasan ST, Zingg JM, Kwan P, Noble T, Smith D, Meydani M. 2014; Curcumin modulation of high fat diet-induced atherosclerosis and steatohepatosis in LDL receptor deficient mice. Atherosclerosis. 232:40–51. DOI: 10.1016/j.atherosclerosis.2013.10.016. PMID: 24401215.
Article32. Cekmen M, Ilbey YO, Ozbek E, Simsek A, Somay A, Ersoz C. 2009; Curcumin prevents oxidative renal damage induced by acetaminophen in rats. Food Chem Toxicol. 47:1480–1484. DOI: 10.1016/j.fct.2009.03.034. PMID: 19345714.
Article33. Zhang J, Tang L, Li GS, Wang J. 2018; The anti-inflammatory effects of curcumin on renal ischemia-reperfusion injury in rats. Ren Fail. 40:680–686. DOI: 10.1080/0886022X.2018.1544565. PMID: 30741618. PMCID: PMC6282432.
Article34. Ibrahim SG, El-Emam SZ, Mohamed EA, Abd Ellah MF. 2020; Dimethyl fumarate and curcumin attenuate hepatic ischemia/reperfusion injury via Nrf2/HO-1 activation and anti-inflammatory properties. Int Immunopharmacol. 80:106131. DOI: 10.1016/j.intimp.2019.106131. PMID: 31981960.
Article35. Yeh CH, Chen TP, Wu YC, Lin YM, Jing Lin P. 2005; Inhibition of NFkappaB activation with curcumin attenuates plasma inflammatory cytokines surge and cardiomyocytic apoptosis following cardiac ischemia/reperfusion. J Surg Res. 125:109–116. DOI: 10.1016/j.jss.2004.11.009. PMID: 15836859.36. Mokhtari-Zaer A, Marefati N, Atkin SL, Butler AE, Sahebkar A. 2018; The protective role of curcumin in myocardial ischemia-reperfusion injury. J Cell Physiol. 234:214–222. DOI: 10.1002/jcp.26848. PMID: 29968913.
Article37. Boyanapalli SS, Kong AT. 2015; "Curcumin, the King of Spices": epigenetic regulatory mechanisms in the prevention of cancer, neurological, and inflammatory diseases. Curr Pharmacol Rep. 1:129–139. DOI: 10.1007/s40495-015-0018-x. PMID: 26457241. PMCID: PMC4596544.
Article38. Jankauskas SS, Pevzner IB, Andrianova NV, Zorova LD, Popkov VA, Silachev DN, Kolosova NG, Plotnikov EY, Zorov DB. 2017; The age-associated loss of ischemic preconditioning in the kidney is accompanied by mitochondrial dysfunction, increased protein acetylation and decreased autophagy. Sci Rep. 7:44430. DOI: 10.1038/srep44430. PMID: 28294175. PMCID: PMC5353572.
Article39. Zager RA, Johnson AC, Becker K. 2011; Acute unilateral ischemic renal injury induces progressive renal inflammation, lipid accumulation, histone modification, and "end-stage" kidney disease. Am J Physiol Renal Physiol. 301:F1334–F1345. DOI: 10.1152/ajprenal.00431.2011. PMID: 21921025. PMCID: PMC3233867.
Article40. Bomsztyk K, Flanagin S, Mar D, Mikula M, Johnson A, Zager R, Denisenko O. 2013; Synchronous recruitment of epigenetic modifiers to endotoxin synergistically activated Tnf-α gene in acute kidney injury. PLoS One. 8:e70322. DOI: 10.1371/journal.pone.0070322. PMID: 23936185. PMCID: PMC3728219.
Article41. Evankovich J, Cho SW, Zhang R, Cardinal J, Dhupar R, Zhang L, Klune JR, Zlotnicki J, Billiar T, Tsung A. 2010; High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J Biol Chem. 285:39888–39897. DOI: 10.1074/jbc.M110.128348. PMID: 20937823. PMCID: PMC3000970.
Article42. Li J, Chen P, Sinogeeva N, Gorospe M, Wersto RP, Chrest FJ, Barnes J, Liu Y. 2002; Arsenic trioxide promotes histone H3 phosphoacetylation at the chromatin of CASPASE-10 in acute promyelocytic leukemia cells. J Biol Chem. 277:49504–49510. DOI: 10.1074/jbc.M207836200. PMID: 12388546.
Article43. Yan X, Pan B, Lv T, Liu L, Zhu J, Shen W, Huang X, Tian J. 2017; Inhibition of histone acetylation by curcumin reduces alcohol-induced fetal cardiac apoptosis. J Biomed Sci. 24:1. DOI: 10.1186/s12929-016-0310-z. PMID: 28056970. PMCID: PMC5217636.
Article44. Peng C, Zhang W, Zhao W, Zhu J, Huang X, Tian J. 2015; Alcohol-induced histone H3K9 hyperacetylation and cardiac hypertrophy are reversed by a histone acetylases inhibitor anacardic acid in developing murine hearts. Biochimie. 113:1–9. DOI: 10.1016/j.biochi.2015.03.012. PMID: 25797917.
Article45. Tesch GH, Ma FY, Nikolic-Paterson DJ. 2016; ASK1: a new therapeutic target for kidney disease. Am J Physiol Renal Physiol. 311:F373–F381. DOI: 10.1152/ajprenal.00208.2016. PMID: 27226108.
Article46. Kanellis J, Ma FY, Kandane-Rathnayake R, Dowling JP, Polkinghorne KR, Bennett BL, Friedman GC, Nikolic-Paterson DJ. 2010; JNK signalling in human and experimental renal ischaemia/reperfusion injury. Nephrol Dial Transplant. 25:2898–2908. DOI: 10.1093/ndt/gfq147. PMID: 20368303.
Article47. Wang Y, Wang Y, Luo M, Wu H, Kong L, Xin Y, Cui W, Zhao Y, Wang J, Liang G, Miao L, Cai L. 2015; Novel curcumin analog C66 prevents diabetic nephropathy via JNK pathway with the involvement of p300/CBP-mediated histone acetylation. Biochim Biophys Acta. 1852:34–46. DOI: 10.1016/j.bbadis.2014.11.006. PMID: 25446993. PMCID: PMC4369325.
Article48. Fiorillo C, Becatti M, Pensalfini A, Cecchi C, Lanzilao L, Donzelli G, Nassi N, Giannini L, Borchi E, Nassi P. 2008; Curcumin protects cardiac cells against ischemia-reperfusion injury: effects on oxidative stress, NF-kappaB, and JNK pathways. Free Radic Biol Med. 45:839–846. DOI: 10.1016/j.freeradbiomed.2008.06.013. PMID: 18638545.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sodium butyrate inhibits high glucose-induced inflammation by controlling the acetylation of NF-κB p65 in human monocytes
- Curcumin, COX-2, and Protein p300/CBP
- The Role of Histone Acetylation in Mesenchymal Stem Cell Differentiation
- SP600125, a selective JNK inhibitor, aggravates hepatic ischemia-reperfusion injury
- Curcumin protects against the intestinal ischemia-reperfusion injury: involvement of the tight junction protein ZO-1 and TNF-alpha related mechanism